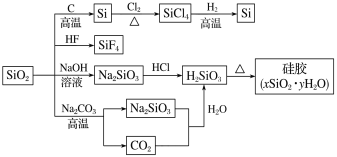
��Ŀ����
����Ŀ��ʵ����Ҫ����1000 mL 2 mol/L NaOH��Һ����ش��������⣺
(1)�����Ǽ���ʵ���г��õ�������
A. 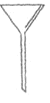 B.
B. ![]() C.
C. D.
D.![]()
�������A������__________
�����ƹ���������Ҫʹ�õĻ�ѧ������______(����������ĸ)��
(2)��������ƽ��ȡ�������ƣ�������Ϊ______g��
(3)������Ҫ�����������ȷ˳����_________(�����)��
�ٳ�ȡһ���������������ƣ������ձ��У�����������ˮ�ܽ⣻
�ڼ�ˮ��Һ��������ƿ���̶�����1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�۴���ȴ�����º���Һת�Ƶ�100 mL ����ƿ�У�
�ܸǺ�ƿ�����������µߵ���ҡ�ȣ�
��������������ˮϴ���ձ��ڱںͲ�����2~3�Σ�ϴ��Һת�Ƶ�����ƿ�С�
(4)��������У���ʹ������ҺŨ��ƫ�ߵ���___________(�����)��
A.����ʱ�۲�Һ�温�� B.û�н��������IJ��������
C.���������������ѳ��� D.����ƿʹ��ǰ�ڱ�մ��ˮ��
���𰸡�©�� AC 8.0g �٢ۢݢڢ� A
��������
(3) ����һ�����ʵ���Ũ�ȵ���Һ��ʵ�鲽����������㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��
(4)����![]() ������
������
(1)�ٸ�������A��ͼʾ������A��������©��������Һʱ����Ҫ����Һת����100mL����ƿ�У�����ʱ����Ҫ�õ�����ιܣ�����Ҫ�õ�©����Բ����ƿ������Ҫ�Ļ�ѧ������AC��
(2)����100mL 2mol��L��1NaOH��Һ������ҪNaOH������m=cVM=2mol��L��1��0.1L��40g��mol��1=8.0g��
(3) ����һ�����ʵ���Ũ�ȵ���Һ��ʵ�鲽����������㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�������Ϊ�ܽ⣬�����Ϊ���ݣ������Ϊ��Һ�������Ϊҡ�ȣ������Ϊϴ�ӣ�����ȷ����Ϊ�٢ۢݢڢܣ�
(4)A������ʱ����Һ�棬��Һ���ƫС�����Ũ��ƫ�ߣ�A�������⣻
B��û��ϴ���ձ��Ͳ��������в�������û��ת��������ƿ�������ʵ���ƫС��Ũ��ƫС��B���������⣻
C�����������������ѳ��⣬���������������ƺ�ˮ�����������Ƴ���ƫС�����Ũ��ƫС��C���������⣻
D������ƿʹ��ǰ��ˮ�飬��ʵ����û��Ӱ�죬D���������⣻
��ѡA��

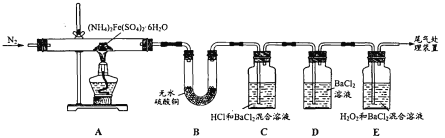
����Ŀ�������ҹ����гɹ����漰�����У���Ҫ�ɷ�Ϊͬ����Ԫ���γɵ����ǽ������ϵ���
|
|
|
|
A��4.03�״�ھ�̼���跴�侵 | B��2022�궬�»�۰����ٻ��� | C�������ε�Ų���̼������������ | D�������ö������ѺϽ�ɸ���� |
A. AB. BC. CD. D
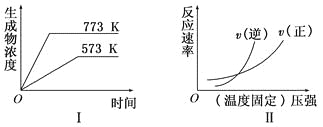
����Ŀ���±���ʾ�ϳɰ���Ӧ��N2+3H2![]() 2NH3���ڲ�ͬ�����´ﵽƽ��ʱ������а��ĺ���[��ʼʱv��N2����v��H2��==1��3]��
2NH3���ڲ�ͬ�����´ﵽƽ��ʱ������а��ĺ���[��ʼʱv��N2����v��H2��==1��3]��
|
|
|
|
|
|
200 | 0.153 | 0.815 | 0.899 | 0.954 | 0.988 |
300 | 0.022 | 0.520 | 0.710 | 0.842 | 0.926 |
400 | 0.004 | 0.251 | 0.470 | 0.652 | 0.798 |
�����ϱ����ݣ��ش��������⣺
��1��200����100MPaʱ��ƽ�������а��ĺ����Ѵ�0.988�������������ѹǿ______
����������������������ʹƽ�������а��ĺ�������1�������ǣ�___________________________________________________________________________________________��
��2����ʹƽ�������а��ĺ���������ɲ�ȡ�Ĵ�ʩ�У�____________________��
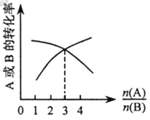
��3����ʹƽ�������а��ĺ���Ϊ0.710����ѡ��ķ�Ӧ����ӦΪ��____________��