��Ŀ����
15�����Ϲ����к���ƻ���ᣬƻ���ᾭ�ۺ����ɾ�ƻ���ᣮ��֪����1��0.1molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������
��2��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��
��3��
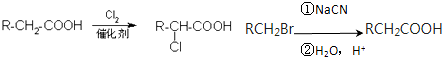
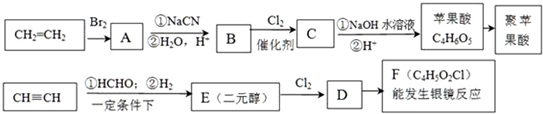
��ش�
��1��д��B�Ľṹ��ʽHOOCCH2CH2COOH��
��2��E�ĺ˴Ź�������������壬��������Ϊ1��2��2��E�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��
��3��д��ƻ�������������ŵ������ǻ����Ȼ���Fת����ƻ������ܷ����ķ�Ӧ����������Ӧ��ȡ����Ӧ��ˮ�ⷴӦ��
��4��д����ƻ���������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ
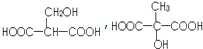 ��
����5��д��F������������Һ��Ӧ�Ļ�ѧ����ʽOHCCH2CHClCHO+4Ag��NH3��2OH$\stackrel{��}{��}$NH4OOCCH2CHClCOONH4+4Ag��+6NH3+2H2O��
��6��д��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽHOOCCH2CHClCOOH+3NaOH$\stackrel{��}{��}$NaOOCCH2CH��OH��COONa+NaCl+2H2O��
��7����ƻ����������õ����������ԣ�����Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽ
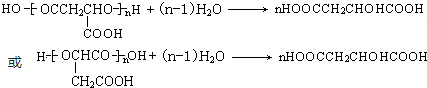 ��
��
���� ƻ�������ʽΪC4H6O5��0��l molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������������̼�����ʵ���Ϊ0.2mol����1molƻ���Ậ2mol-COOH��ƻ������ˮ������ʹ��ˮ��ɫ�IJ�����ƻ����ķ���ʽ֪��ƻ����Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��ƻ����������Ӧ���еľۺ����ɾ�ƻ���ᣨPMLA������ṹΪ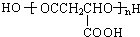 ����ϩ���巢���ӳɷ�Ӧ����A����AΪBrCH2CH2Br��A������Ϣ��Ӧ���ȷ���ȡ����Ӧ���ٷ���ˮ������B����BΪHOOCH2CH2COOH��B����������ȡ����Ӧ����C�����ƻ����Ľṹ��֪��CΪHOOCCH2CH��Cl��COOH����F�ķ���ʽ��֪����Ȳ��HCHO�����������ӳɷ�Ӧ����E��E�ĺ˴Ź�������������壬��������Ϊ1��2��2��EΪHOCH2CH2CH2CH2OH��F�ܷ�Ӧ������Ӧ������ȩ��-CHO��F����ϵ��ת������ƻ���ᣬ���ƻ����Ľṹ��F�ķ���ʽ����֪FΪOHCCH2CH��Cl��CHO�����C��F�Ľṹ��֪��DΪHOCH2CH2CH��Cl��CH2OH�������л���Ľṹ�����ʽ��
����ϩ���巢���ӳɷ�Ӧ����A����AΪBrCH2CH2Br��A������Ϣ��Ӧ���ȷ���ȡ����Ӧ���ٷ���ˮ������B����BΪHOOCH2CH2COOH��B����������ȡ����Ӧ����C�����ƻ����Ľṹ��֪��CΪHOOCCH2CH��Cl��COOH����F�ķ���ʽ��֪����Ȳ��HCHO�����������ӳɷ�Ӧ����E��E�ĺ˴Ź�������������壬��������Ϊ1��2��2��EΪHOCH2CH2CH2CH2OH��F�ܷ�Ӧ������Ӧ������ȩ��-CHO��F����ϵ��ת������ƻ���ᣬ���ƻ����Ľṹ��F�ķ���ʽ����֪FΪOHCCH2CH��Cl��CHO�����C��F�Ľṹ��֪��DΪHOCH2CH2CH��Cl��CH2OH�������л���Ľṹ�����ʽ��
��� �⣺ƻ�������ʽΪC4H6O5��0��l molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������������̼�����ʵ���Ϊ0.2mol����1molƻ���Ậ2mol-COOH��ƻ������ˮ������ʹ��ˮ��ɫ�IJ�����ƻ����ķ���ʽ֪��ƻ����Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��ƻ����������Ӧ���еľۺ����ɾ�ƻ���ᣨPMLA������ṹΪ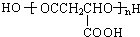 ����ϩ���巢���ӳɷ�Ӧ����A����AΪBrCH2CH2Br��A������Ϣ��Ӧ���ȷ���ȡ����Ӧ���ٷ���ˮ������B����BΪHOOCH2CH2COOH��B����������ȡ����Ӧ����C�����ƻ����Ľṹ��֪��CΪHOOCCH2CH��Cl��COOH����F�ķ���ʽ��֪����Ȳ��HCHO�����������ӳɷ�Ӧ����E��E�ĺ˴Ź�������������壬��������Ϊ1��2��2��EΪHOCH2CH2CH2CH2OH��F�ܷ�Ӧ������Ӧ������ȩ��-CHO��F����ϵ��ת������ƻ���ᣬ���ƻ����Ľṹ��F�ķ���ʽ����֪FΪOHCCH2CH��Cl��CHO�����C��F�Ľṹ��֪��DΪHOCH2CH2CH��Cl��CH2OH��
����ϩ���巢���ӳɷ�Ӧ����A����AΪBrCH2CH2Br��A������Ϣ��Ӧ���ȷ���ȡ����Ӧ���ٷ���ˮ������B����BΪHOOCH2CH2COOH��B����������ȡ����Ӧ����C�����ƻ����Ľṹ��֪��CΪHOOCCH2CH��Cl��COOH����F�ķ���ʽ��֪����Ȳ��HCHO�����������ӳɷ�Ӧ����E��E�ĺ˴Ź�������������壬��������Ϊ1��2��2��EΪHOCH2CH2CH2CH2OH��F�ܷ�Ӧ������Ӧ������ȩ��-CHO��F����ϵ��ת������ƻ���ᣬ���ƻ����Ľṹ��F�ķ���ʽ����֪FΪOHCCH2CH��Cl��CHO�����C��F�Ľṹ��֪��DΪHOCH2CH2CH��Cl��CH2OH��
��1����������ķ�����֪��B�Ľṹ��ʽΪHOOCCH2CH2COOH��
�ʴ�Ϊ��HOOCCH2CH2COOH��
��2����������ķ�����֪��EΪHOCH2CH2CH2CH2OH��
�ʴ�Ϊ��HOCH2CH2CH2CH2OH��
��3��ƻ����Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��ƻ�������������ŵ�����Ϊ�ǻ����Ȼ���FΪOHCCH2CH��Cl��CHO���Ƚ�F��ƻ����Ľṹ��ʽ��֪��Fת����ƻ������ܷ����ķ�Ӧ����Ϊ������Ӧ��ȡ����Ӧ��ˮ�ⷴӦ��
�ʴ�Ϊ���ǻ����Ȼ���������Ӧ��ȡ����Ӧ��ˮ�ⷴӦ��
��4����ƻ���������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪ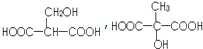 ��
��
�ʴ�Ϊ��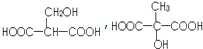 ��
��
��5��FΪOHCCH2CH��Cl��CHO��F������������Һ��Ӧ�Ļ�ѧ����ʽOHCCH2CHClCHO+4 Ag��NH3��2OH$\stackrel{��}{��}$NH4OOCCH2CHClCOONH4+4Ag��+6NH3+2H2O��
�ʴ�Ϊ��OHCCH2CHClCHO+4 Ag��NH3��2OH$\stackrel{��}{��}$NH4OOCCH2CHClCOONH4+4Ag��+6NH3+2H2O��
��6��CΪHOOCCH2CH��Cl��COOH��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪHOOCCH2CHClCOOH+3NaOH$\stackrel{��}{��}$NaOOCCH2CH��OH��COONa+NaCl+2H2O��
�ʴ�Ϊ��HOOCCH2CHClCOOH+3NaOH$\stackrel{��}{��}$NaOOCCH2CH��OH��COONa+NaCl+2H2O��
��7����ƻ��������������ˮ��Ļ�ѧ����ʽΪ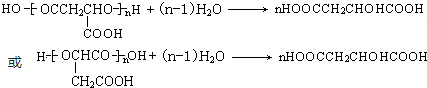 ��
��
�ʴ�Ϊ��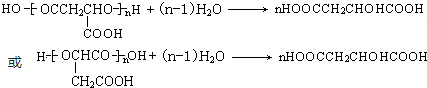 ��
��
���� ���⿼���л�����ƶ���ϳɡ��Ը�����Ϣ�����á������ŵ�������ת������ȷƻ������������ƶ���ṹ�ǽ����Ĺؼ���ע����ݷ�Ӧ��Ϣ�����ƶϣ���Ŀ�Ѷ��еȣ�

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д� ������ȷ˳���ǣ�������
������ȷ˳���ǣ�������| A�� | �٢ݢڢۢ� | B�� | �٢ڢۢܢ� | C�� | �ڢۢݢ٢� | D�� | �ڢۢݢ٢� |
 ���л���X�ļ���ʽΪ
���л���X�ļ���ʽΪ ������˵���в���ȷ���ǣ�������
������˵���в���ȷ���ǣ�������| A�� | X�Ļ�ѧʽΪC8H8 | |
| B�� | �л���Y��X��ͬ���칹�壬�����ڷ���������Y�Ľṹ��ʽΪ | |
| C�� | X��ʹ���Ը��������Һ��ɫ | |
| D�� | X��������H2��һ�������·�Ӧ�����ɻ�״�ı�����Z��Z��һ�ȴ�����4�� |
| A�� | 92.1% | B�� | 84.6% | C�� | 92.3% | D�� | 84.2% |
| A�� | NH3��H2O | B�� | HCl��KCl | C�� | H2O��H2O2 | D�� | NaCl��NaOH |
| A�� | ����������ȡ��Һ | |
| B�� | ������Һ�����ܽ����Ҫ�ɷ��DZ��״� | |
| C�� | �������������ò�Ʒ���DZ��״� | |
| D�� | ��������˵õ���Ʒ���DZ������ |
| A�� | ��ˮ��Һ�У�C6H5COOH�ĵ��뷽��ʽΪ��C6H5COOH?C6H5COO-+H+ | |
| B�� | 0.1mol•L-1C6H5COONa��Һ������Ũ�ȴ�С��ϵΪ��c��Na+����c��C6H5COO-����c��OH-����c��H+�� | |
| C�� | C6H5COONa��C6H5COOH�Ļ����Һ�����ԣ���c��Na+��=0.1mol•L-1����c��Na+��=c��C6H5COO-����c��OH-��=c��H+�� | |
| D�� | ��Ũ�ȵ�C6H5COONa��CH3COONa����Һ�У�ǰ��������Ũ��С�ں��� |

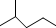 ��ʾ�ķ���ʽC6H14��������2-�����飮
��ʾ�ķ���ʽC6H14��������2-�����飮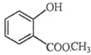 �к��еĹ����ŵ�����Ϊ�ǻ���������
�к��еĹ����ŵ�����Ϊ�ǻ���������