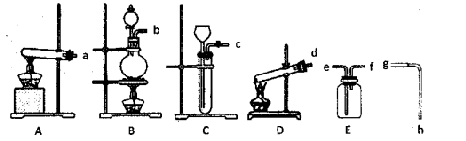
��Ŀ����
���ͷ���һ�ֻ�ѧ���ɼ�,����С�մ���(̼�����)�������е�����������ɡ�ijС��Ϊ̽����ͬƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ�,��������ʵ�顣
��������衿
(1)����1:��С�մ�ͳ������
����2:��С�մ���������
����3:�������������
�����������̡�
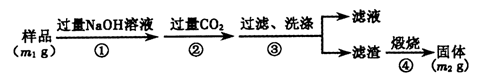
Ϊ̽����Ʒ�Ƶķ��ͷ۵ijɷ�,ijͬѧ�������ʵ��,�õ���������: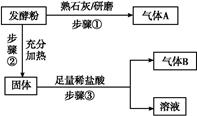
(2)��ϲ���١��۷���,����AΪ��������,�÷��ͷ۵ijɷ�Ϊ�������������������� (�ѧʽ)��
(3)������ٺ͢ڲ�������(����Ҳ��ͬ),�������������ϡ�����Ϊ�����Ȼ�����Һ,�۲쵽�а�ɫ��������,�ܷ�ȷ�����ͷ۵ijɷֲ�˵������:���������������������������������������������������������������������������������������������������������������������� ����
(4)��Ʒ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2���,�������ʵ����֤��
ʵ����������Ʒ��ѡ,��ѡ�Լ�:ϡ���ᡢ0.1 mol/L NaOH��Һ
д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1:ȡ������Ʒ���������������,����Һ�ֳ�����,�ֱ�װ��A��B�Թ��� | |
| ����2:���������������������������������������� ���������������������������������������� | ������������,֤����Na+,���ͷ�����NaHCO3 |
| ����3:���������������������������������������� ���������������������������������������� | ������������,��ϲ���2�еĽ���,����2���� |
(1)���ۺ�������(2)����(��NH3)�� NaHCO3��NH4HCO3(д����Ҳ����)
(3)����,��Ϊ����NaHCO3�ֽ��Na2CO3,Na2CO3��KAl(SO4)2������BaCl2��Һ��Ӧ���ɰ�ɫ������
(4)����2:�ýྻ�IJ�˿պȡA�е���Һ,�ھƾ��ƻ���������,�۲���ɫ ��ɫ�ʻ�ɫ ����3:������1�� ��B�Թ�����εμ�0.1 mol/L NaOH��Һ
������2����պȡA�е���Һ(��պȡB��Һ),�ھƾ��ƻ��������������а�ɫ��������(���Ȳ�����ɫ����������ܽ�),֤�����ͷ���������
����ɫ�ܲ����۲���ɫ����ɫ,֤�����ͷ��к�������
����

ij��ѧС���Ա�����Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе����±���
| ���� | �״� | ������ | ��������� |
| �е�/�� | 64��7 | 249 | 199��6 |
�ϳɱ���������ֲ�Ʒ
��Բ����ƿ�м���12��2g�������20ml�״����ܶ�Լ0��79g��mL-1��,��С�ļ���3mLŨ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ����
��1��Ũ����������� ��
|
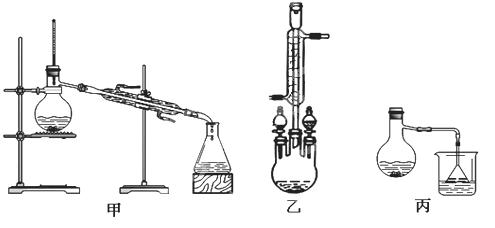
�ֲ�Ʒ�ľ���
��3������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ��������������̽��о��ƣ����������ͼд���������������ơ������� ������ ��
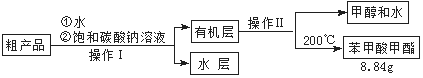
��4���ܷ���NaOH��Һ���汥��̼������Һ�� ����ܡ�����
������ԭ�� ��
��5��ͨ�����㣬����������IJ����� ��
��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�
NaBr + H2SO4 �� NaHSO4 + HBr
CH3CH2OH + HBr CH3CH2Br + H2O
CH3CH2Br + H2O
ij����С������ʵ�����Ʊ��������װ������ͼ���������±���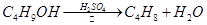
| ���� ���� | �Ҵ� | ������ | 1��2-�������� | ���� | Ũ���� |
| �ܶ�/g��cm-3 | 0.79 | 1.46 | 2.2 | 0.71 | 1.84 |
| �۵㣨�棩 | ��130 | ��119 | 9 | ��116 | 10 |
| �е㣨�棩 | 78.5 | 38.4 | 132 | 34.6 | 338 |
| ��ˮ�е��ܽ�ȣ�g/100gˮ�� | ���� | 0.914 | 1 | 7.5 | ���� |
��ش��������⡣
��1������ҩƷ֮ǰ�����IJ�����:_________________��ʵ����е�;��������δ�������Ƭ���䴦���ķ�����__________________��
��2��װ��B�������dz���ʹ���������������һ��Ŀ����_____________���¶ȼƵ��¶�Ӧ������_____________֮�䡣
��3����Ӧʱ�п�������SO2��һ�ֺ���ɫ���壬��ѡ������������Һ��ȥ�����壬�йص����ӷ���ʽ��___________��______________���˲�������___________����д�����������ƣ��н��У�ͬʱ���з��롣
��4��ʵ���в���80%���ᣬ��������98%Ũ���ᣬһ������Ϊ�˼��ٸ���Ӧ����һ������Ϊ��_______________________��
��5���ֲ�Ʒ�к��е���Ҫ�л�Һ��������_____________��Ϊ��һ���Ƶô����������飬�Դֲ�Ʒ����ˮϴ�ӡ���Һ���ټ�����ˮCaCl2������______________������
����ͪ��һ����Ҫ���л�����ԭ�ϡ�ʵ���Һϳɻ���ͪ�ķ�Ӧ���£�
�������ͻ���ͪ�IJ����������ʼ��±���
| ���� | ��Է������� | �е�(��) | �ܶ�(g��cm��3��20 ��) | �ܽ��� | |
| ������ | 100 | 161.1 | 0.9624 | ������ˮ���� | |
| ����ͪ | 98 | 155.6 | 0.9478 | ����ˮ���������� | |
����20mL������������Na2Cr2O7������Ļ��Һ��ַ�Ӧ���Ƶ���Ҫ������ͪ��ˮ�Ĵֲ�Ʒ��Ȼ����з����ᴿ������Ҫ������(δ����)��
A������ȥ���Ѻ��ռ�151��~156�����
B��ˮ��������(���ѷе�34.6�棬��ȼ��)��ȡ����ȡҺ�����л���
C������
D����Һ���м���NaCl���������ͣ����ã���Һ
E��������ˮMgSO4���壬��ȥ�л���������ˮ
�ش��������⣺
��1���������ᴿ�������ȷ˳���� ��
��2��b��ˮ����������ȡ��Ŀ���� ��
��3�����¹�����ȡ��Һ�����������У�����ȷ���� ��
A��ˮ��Һ�м������ѣ�ת������Һ©�������ϲ���������ͼ������

B�����κ����Һ©���ϿڵIJ���������
C�����������������ֳַ�©������Һ��ֲ�
D����Һʱ�����Ƚ��Ͽڲ������������ϵİ��۶�©���ϵ�С�ף��ٴ��������²�Һ��ȫ������ʱ���ٴ��Ͽڵ����ϲ�Һ��
��4������������D�У�����NaCl����������� ����������ѵIJ����в��õļ��ȷ�ʽΪ ��
��5���������ʱ��һ��ʱ�����δͨ����ˮ��Ӧ��ȡ����ȷ������ ��
��6���ָ�������ʱ������õ�����Ʒ���Ϊ12mL����ͪ�IJ���Լ�� ��
������ʾ��þ�뱥��̼��������Һ��Ӧ������������Ͱ�ɫ�����ijͬѧ���������ʵ�鷽��̽����Ӧԭ������֤���
(1)�������
ʵ�����ɰֽ��ȥþ����������Ĥ���������ʢ���������з�̪�ı���̼��������Һ���Թ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz���졣
��ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ________��
�²�2����ɫ���������ΪMgCO3��
�²�3����ɫ����������Ǽ�ʽ̼��þ[xMgCO3��yMg(OH)2]��
(2)��ƶ���ʵ��ȷ�����ﲢ��֤�²⣺
| ʵ����� | ʵ�� | ʵ������ | ���� |
| ʵ��� | ��ʵ������� �����������ȼ | �ܰ���ȼ�ա��� ������ɫ���� | ����ɷ�Ϊ __��__ |
| ʵ��� | ȡʵ����еİ� ɫ�����ϴ�ӣ� ��������__��__ | __��__ | ��ɫ��������ܺ���MgCO3 |
| ʵ��� | ȡʵ����еij� ��Һ�������м��� ����CaCl2ϡ��Һ | ������ɫ���� | ��Һ�д���__��__ |
(3)Ϊ��һ��ȷ��ʵ���IJ����ƶ���ʵ�鷽������ͼ��ʾ��
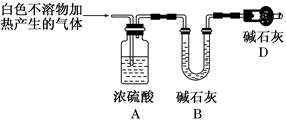
��ȡʵ��������ø�������İ�ɫ������22.6 g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������װ��A��B�С�ʵ��ǰ��װ��A����1.8 g��װ��B����8.8 g����ȷ����ɫ������Ļ�ѧʽΪ_____________________________________________��
(4)���ϻ�ѧ����ͻ�ѧƽ���ƶ�ԭ������Mg�ͱ���NaHCO3��Һ��Ӧ�����������ݵ�ԭ��______________________________________________________________
________________________________________________________________________��
����ͭ���仯����һ�㶼�����������ɫ����
| Cu | Cu2O | CuO | Cu��OH��2 | CuSO4��5H2O |
| ��ɫ�����Ϻ�ɫ�� | ��ɫ����ש��ɫ�� | ��ɫ | ��ɫ | ��ɫ |
ijѧУѧϰС���Ϊ���ʵ������H2��ԭCuO���ú�ɫ�������Ƿ���Cu2O��������������о���
��.�������ϵó�������Ϣ��
��Cu2O���ڼ��������
�ڸ�������CuO����Cu2O��
��Cu2O�������������ܷ�����Ӧ��Cu2O��2H��=Cu��Cu2����H2O��
��.���ʵ�鷽����
����1��ȡ�ú�ɫ��������������ϡ�����У��۲���Һ��ɫ�仯��
����2��ȡ�ú�ɫ��������������ϡ�����У��۲���Һ�Ƿ����ɫ��
����3���Ƶø�������������Ϊa g��ȡ��ɫ�������������гƵ�������Ϊb g���ڿ����и��������������㶨���Ƶ����������Ϊc g��
��1��д��Cu2O��ϡ���ᷴӦ�Ļ�ѧ����ʽ��_______________________��
��2���������۷���1�ͷ���2�ĺ����ԣ����������ɣ�
����1��_______________________��
����2��_______________________��
��3������3�У���ȷ�Ϻ�ɫ�����к���Cu2O����a��b��c�Ĺ�ϵΪ________���ڸ�ʵ�鷽��������Ӧ����________�γ�����
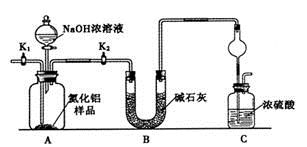
��.ѧϰС����������µ�̽������������ݸ��������ˮ����ͭ�Ƿ�����жϺ�ɫ�������Ƿ���Cu2O��װ����ͼ��ʾ��

��4����̽�������м������巢��װ�������Եķ���Ϊ________��˵����������������ͽ��ۣ���
��5��Ϊȷ��̽���Ŀ�ѧ�������Ͱ�ȫ������Ϊʵ���л�Ӧ��ȡ�Ĵ�ʩ��____������ţ���
A������������װ����Ӳ�ʲ�����֮������һ������װ��
B������ǰ���ž�װ���еĿ���
C����ʢ����ˮ����ͭ�ĸ���ܺ�������һ��װ�м�ʯ�ҵĸ����


 2KCl��3O2��
2KCl��3O2��