��Ŀ����
6����������ڱ��е�һ���֣�����A-I�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش����⣺| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| 1 | A | |||||||
| 2 | D | E | G | I | ||||
| 3 | B | C | F | H |
��2��������עA-IԪ�أ�����HԪ�ص�����ϼ���+7���γɻ�������������Ԫ����C��
��3��D����OԪ���γ����ֻ�����ֱ���CO��CO2
��4����B��C��D��E��F��G��H�У�ԭ�Ӱ뾶������Na
��5��A��E�γɻ�����Ļ�ѧʽNH3�ȣ�
���� ��Ԫ�������ڱ���λ�ã���֪AΪ�⡢BΪNa��CΪAl��DΪ̼��EΪ����FΪ�ס�GΪ����HΪCl��IΪNe��
��1��ϡ�����廯ѧ�������ȶ���FԪ��ֻ�и��۶�û�����ۣ�ͬ�����������Ԫ�ؽ����Լ������ǽ�������ǿ��ͬ�������϶���Ԫ�ؽ�������ǿ���ǽ����Լ������ǽ�����Խǿ������������Խǿ��������Խǿ�����ʻ�ԭ��Խǿ��
��2������Ԫ����������ϼ۵�����������������Ԫ�ء���Ԫ�س��⣩��̼Ԫ������л���γɵ�����������ࣻ
��3��̼Ԫ������Ԫ���γɵĻ�������һ����̼��������̼��
��4��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
��5��A��E�γɻ������а����ȣ�
��� �⣺��Ԫ�������ڱ���λ�ã���֪AΪ�⡢BΪNa��CΪAl��DΪ̼��EΪ����FΪ�ס�GΪ����HΪCl��IΪNe��
��1��ϡ������Neԭ�������Ϊ�ȶ��ṹ����ѧ��������ã�FԪ��ֻ�и��۶�û�����ۣ�ͬ�����������Ԫ�ؽ����Լ������ǽ�������ǿ��ͬ�������϶���Ԫ�ؽ�������ǿ���ǽ����Լ���������Ԫ����FԪ�طǽ�������ǿ��NaԪ�ؽ�������ǿ����F2����������ǿ��Na�Ļ�ԭ����ǿ���ʴ�Ϊ��Ne��F��F2��Na��
��2������Ԫ����������ϼ۵�����������������Ԫ�ء���Ԫ�س��⣩����H����������ϼ�Ϊ+7��̼Ԫ������л���γɵ�����������࣬
�ʴ�Ϊ��+7��C��
��3��̼Ԫ������Ԫ���γɵĻ�������CO��CO2���ʴ�Ϊ��CO��CO2��
��4��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶������B��C��D��E��F��G��H�У�ԭ�Ӱ뾶������Na���ʴ�Ϊ��Na��
��5��A��E�γɻ�������NH3�ȣ��ʴ�Ϊ��NH3�ȣ�
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��Ƚϻ�����ּ�ڿ���ѧ���Ի���֪ʶ���������գ�

| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ��������Һ�зֱ���뱥������狀ʹ���Ǧ��Һ | ���й������� | ���������� |
| B | ��ˮ�зֱ���뱽�Ӻͻ���ϩ | ��ˮ����ɫ | �������ӳɷ�Ӧ |
| C | ij��ɫ����ͨ����ˮ�� | ��ˮ��ɫ | ������һ����C2H4 |
| D | ����ˮ��������ϡ�����У� ������Һ���ü�������� | �ж����ЧӦ | �й��ὺ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���ǡ���ѿ�ǻ�Ϊͬ���칹�� | |
| B�� | ���õ����ʵ���ɫ��Ӧ�����ֵ����� | |
| C�� | ��Na2CO3��Һ��������CH3COOH��CH3COOCH2CH3 | |
| D�� | ��֬�����Ի���������¾��ܷ���ˮ�ⷴӦ���Ҳ��ﲻͬ |
| A�� | ��ˮ����� | B�� | ˮ�� | C�� | ���������� | D�� | ��ͨ���� |
��1��������һ����Ԫ��ǿ�ᣬ����Һ�и��ֺ�P���ӵİٷ������ģ�����Һ��pH��ϵ��ͼ��������ĵڶ�������ƽ�ⳣ��ԼΪ10-7��Na2HPO4��Һ��pHԼΪ10��˵��HPO4-ˮ��̶ȴ��ڵ���̶ȣ����������ˮ��̶������ͣ���Na2HPO4��Һ�������غ�ʽΪc��Na+��=2[c��H3PO4��+c��H2PO4-��+c��HPO42-��+c��PO43-��]��
��2�����ᣨ��HAC����ʾ����һ����Ҫ�Ļ���ԭ��Ҳ�����ܼ���HClO4��HCl��HNO3��H2SO4����ˮ�����ж������ᣬ�������µ���ƽ�⣨���볣����Ϊ�����µ���ֵ����
HClO4+HAC�TH2AC++ClO4-K�T1.6��10-5
HCl+HAC�TH2AC++Cl-K�T1.6��10-9
HNO3+HAC�TH2AC++NO3-K�T4.2��10-10
H2SO4+HAC�TH2AC++HSO4-K�T6.3��10-9
��H2AC+������Ϊ����������ӣ����������ܼ�ʱ��HClO4��HCl��HNO3��H2SO4��������ǿ����������ΪHClO4��H2SO4��HCl��HNO3����0.01mol/L HNO3��������Һ�У�c��NO3-��=2��10-6������������һλ��Ч���֣���
����֪�������ˮ�еĵ��볣����25�棩
| �� | HCN | HAc | HF | H2SO3 |
| Ka | 4.9��10-10 | 1.75��10-5 | 3.5��10-4 | K1=1.5��10-2 |
��3�����ᣨŨ�����Ũ����Ļ����dz��õ������Լ������к���������Ӧ���м���NO2+���䷴Ӧ�ǣ�2H2SO4+HNO3�T[NO2]+[HSO4]-+[H3O+][HSO4]-�������кͷ�Ӧ��ԭ���жϴ˷�Ӧ��HNO3��Ϊ�NO2+��CO2��N2O��N3-��-OCN���ѧʽ����ԭ�����͵�������ͬ���ṹ���ƣ�H3O+�Ŀռ乹��Ϊ�����Σ�

| A�� | S | B�� | Fe | C�� | Si | D�� | Cl |
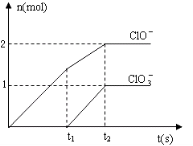 ��һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��C1O-��C1O3-���ֺ���Ԫ�ص����ӣ�����C1O-��C1O3-�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
��һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��C1O-��C1O3-���ֺ���Ԫ�ص����ӣ�����C1O-��C1O3-�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��