��Ŀ����
��ѧ�ҷ��֣����ܻ�ѧ��Ӧ��һ����ɻ�ּ�����ɣ��÷�Ӧ����ЧӦ����ͬ�ġ���֪��25�桢105 Paʱ��C(ʯī) + O2(g) = CO2(g) ��H =" -" 393. 5 kJ��mol-1
2CO(g) + O2(g) = 2CO2(g) ��H =" -" 283.0 kJ��mol-1
����˵����ʽ��ȷ����
2CO(g) + O2(g) = 2CO2(g) ��H =" -" 283.0 kJ��mol-1
����˵����ʽ��ȷ����
| A������ͬ�����£�2C(ʯī) + O2(g) = 2CO(g) ��H =" -" 110.5 kJ��mol-1 |
| B��1 molʯī����ȫȼ�գ�����CO2��CO�������ʱ������504.0 kJ |
| C������ͬ�����£�C(ʯī) + CO2(g) = 2CO(g) ��H =" -" 172.5 kJ��mol-1 |
| D����֪���ʯȼ���ȴ���ʯī��ȼ���ȣ���ʯīת��Ϊ���ʯ��Ҫ���� |
D
���������A����е�һ���Ȼ�ѧ����ʽ����2���ټ�ȥ�ڶ����Ȼ�ѧ����ʽ����2C(ʯī) + O2(g) = 2CO(g) ��H =" -" 504 kJ��mol-1���ʴ���B���A��õ����Ȼ�ѧ����ʽ�����е�һ���Ȼ�ѧ����ʽ���ӣ��ٳ���2����C(ʯī) + O2(g) = CO2(g)+ CO(g) ��H =" -" 448.75 kJ��mol-1���ʴ���C����е�һ���Ȼ�ѧ����ʽ��ȥ�ڶ����Ȼ�ѧ����ʽ����C(ʯī) + CO2(g) = 2CO(g) ��H =" -" 676.5 kJ��mol-1���ʴ�����ѡD��
���������⿼����ǻ�ѧ��Ӧ�ȵļ��㣬��Ŀ�Ѷ��У�ע���Ȼ�ѧ����ʽ���мӼ�����Ӧ��Ҫ���Ÿı䡣

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
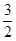 O2(g);��H="+765.2" kJ��mol-1�����ڴ��������ͼ�У�����������Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע��
O2(g);��H="+765.2" kJ��mol-1�����ڴ��������ͼ�У�����������Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע��