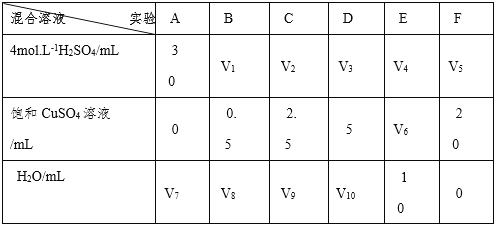
��Ŀ����
����Ŀ����ҵ��ˮ�г�����һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ���˺���������д��������õĴ��������ǻ�ԭ���������÷��Ĺ�������Ϊ��![]()
���е���������ƽ�⣺2 CrO42-(��ɫ)+2H+![]() Cr2O72- (��ɫ)+H2O
Cr2O72- (��ɫ)+H2O
��1�� ��ƽ����ϵ��pH=12������Һ��_______ɫ��
��2�� ��˵����������Ӧ��ƽ��״̬����______________��
a��Cr2O72-��CrO42-��Ũ�Ȳ���
B��2v(Cr2O72-) =v(CrO42-)
C����Һ����ɫ����
��3���������У���ԭlmol Cr2O72-���ӣ���Ҫ_______mol��FeSO47H2O��
��4�����������ɵ�Cr(OH)3����Һ�д������³����ܽ�ƽ�⣺
Cr (OH)3 (s)![]() Cr3+ (aq) +3OH- (aq)
Cr3+ (aq) +3OH- (aq)
�����£�Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH-) = 10-32��Ҫʹ0.01mol/Lc(Cr3+)��ʼ��������Һ��pHӦ����__________����ʹc(Cr3+)����10-5mol/L����Һ��pHӦ����___________��
���𰸡���1������2��ac��3��6��4��4��5
��������
�����������1��c(OH-)����ƽ��2CrO42-(��ɫ)+2H+![]() Cr2O72-(��ɫ)+H2O���ƣ���Һ�ʻ�ɫ��
Cr2O72-(��ɫ)+H2O���ƣ���Һ�ʻ�ɫ��
��2�������ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣻a��Cr2O72-��CrO42-��Ũ�Ȳ��ٸı䣬���ж�ƽ�⣬��a��ȷ��b��2v(Cr2O72-)=v(CrO42-)�������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣬��b����c����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣬��c��ȷ���ʴ�Ϊac��
��3�����ݵ��ӵ�ʧ�غ㣺n(Cr2O72-)��6=n(FeSO47H2O)��1��n(FeSO47H2O)=![]() =6mol��
=6mol��
��4��Ҫʹ0.01mol/Lc(Cr3+)��ʼ��������Һ��c(OH-)=![]() =10-10mol/L����ʱ��ҺpH=4����c(Cr3+)=10-5mol/Lʱ����Һ��c(OH-)=
=10-10mol/L����ʱ��ҺpH=4����c(Cr3+)=10-5mol/Lʱ����Һ��c(OH-)=![]() =10-9mol/L��c(H+)�T
=10-9mol/L��c(H+)�T![]() =10-5mol/L��pH=5����Ҫʹc(Cr3+)����10-5mol/L����Һ��pHӦ����5��
=10-5mol/L��pH=5����Ҫʹc(Cr3+)����10-5mol/L����Һ��pHӦ����5��

����Ŀ����֪������Ϣ����1 mol N2�Ĺ��ۼ���������946 kJ��������1 mol H2�Ĺ��ۼ���������436 kJ���������γ�1 mol NH3�еĻ�ѧ���ͷ�1 173 kJ���������ڽ�һ������N2��H2Ͷ��һ�ܱ������У���һ�������½��з�Ӧ������й��������£�
N2(mol��L��1) | H2(mol��L��1) | NH3(mol��L��1) | |
��ʼʱ | 3 | 3 | 0 |
2sĩ | 2.6 | 1.8 | 0.8 |
��������������ݻش����⣺
(1)��H2��ʾ�÷�Ӧ2 s�ڵ�ƽ����Ӧ����Ϊ________
(2)______(��ܡ����ܡ�)ȷ�ϸ÷�Ӧ2 sĩ�Ѵﻯѧƽ��״̬��
(3)д���÷�Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(4)�������������ɰ����Ĺ���______(��ͷš������ա�)������
����Ŀ���±���Ԫ�����ڱ���һ���֣��١������ijԪ�أ���ش��������⣺
���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
�� | �� | �� | �� | |||||
�� | �� | �� | �� | �� | �� | �� | ||
�� | �� |
��1���ڢ١�����,��ѧ��������õ�Ԫ����ԭ�ӽṹʾ��ͼΪ___________��
��2���ڢ١����У���������ǿ��Ԫ����_________(��Ԫ�ط���)���ڻ������о�ֻ�Ը��۵�Ԫ����__________(��Ԫ�ط���)��
��3���ڢܡ����У�Ԫ�ص�����������Ӧ��ˮ������������ǿ����__________(�����ʻ�ѧʽ,��ͬ)��������ǿ����________��
��4���ڢܡ����У�ԭ�Ӱ뾶��С����__________(��Ԫ�ط���)�������Ӱ뾶��С����_______(�����ӷ���)��
��5���ڢ١��ۡ��ߡ���Ԫ�ص���̬�⻯�������ȶ�����__________(���⻯�ﻯѧʽ)��
��6��д����ҵ���âٵ����ڸ���������ȡ�ߵ��ʵĻ�ѧ����ʽ______________________��