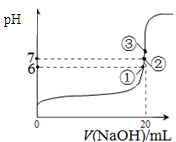
��Ŀ����
X��Y��Z��W��Ϊ10���ӵķ��ӻ����ӡ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣����֮��ת����ϵ��ͼ��ʾ����ش�
��1����ҵ��ÿ��ȡ1molZҪ�ų�46.2 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2����ҵ��ȡZ�Ļ�ѧƽ�ⳣ��K��T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 | ���� |
| K/(mol��L��1)��2 | 4.1��106 | K1 | K2 | ���� |
������������⣺
���ԱȽ�K1��K2�Ĵ�С��K1 K2����д��>����=����<����
�ں��¹̶�����������У����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�����ݵ��� ���������ĸ����
A�������ڸ����ʵ�Ũ��֮��Ϊ��ѧ��������
B����������ܶȱ��ֲ���
C��������ѹǿ���ֲ���
D�����������Է����������ֲ���
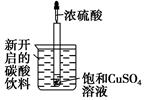
��3��ij��ѧС��ͬѧģ�ҵ������ȡHNO3�������ͼ��ʾװ�ã�����aΪһ���ɳ��������������Ƥ��

��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��B��ŨH2SO4�������� ��
��4��д��Dװ���з�Ӧ�Ļ�ѧ����ʽ ��
��5��a��ͨ����������� ��
��1��N2(g)+3H2 2NH3(g) ��H=��92.4KJ/mol��3�֣�
2NH3(g) ��H=��92.4KJ/mol��3�֣�
(2) �� > ��CD
��3����4NH+5O2 4NO+6H2O ������ˮ�Ͷ���İ���
4NO+6H2O ������ˮ�Ͷ���İ���
(4)3 NO2+H2O=2HNO3+NO��4NO2+O2+2H2O=4HNO3
(5)����NH3��NO
���������������1��5��ԭ�Ӻ˵�10���ӵ���һ��ΪCH4��NH4+NH4+��WΪ��ɫҺ��,10���ӵ���ɫҺ��ΪH2O�����Բ���÷�ӦΪNH4+��OH-��ȡNH3�ķ�Ӧ��XΪNH4+��YΪOH-��ZΪNH3����ҵ��ȡ�����Ļ�ѧ��ӦΪN2+3H2 2NH3��ÿ��ȡ1molNH3Ҫ�ų�46.2kJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2
2NH3��ÿ��ȡ1molNH3Ҫ�ų�46.2kJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2 2NH3(g) ��H=��92.4KJ/mol
2NH3(g) ��H=��92.4KJ/mol
��2������ȡ�����ķ�ӦΪ���ȷ�Ӧ�������¶�������ƽ�������ƶ���ƽ�ⳣ����С������K1>K2
��A����ƽ��ʱ�����ڸ����ʵ�Ũ��֮�Ȳ�һ��Ϊ��ѧ�������ȣ�����B�����ݵ������У�������ܶ�ʼ�ղ��䣬����C�����ŷ�Ӧ�Ľ��У������ѹǿ��С����ƽ��ʱ���ٱ仯����ȷ��D�����ŷ�Ӧ�Ľ��У���������ʵ�����С���������Է�������������ƽ��ʱ���ٱ仯����ȷ����ѡCD��
��3����A�з�����Ӧ�ǰ����Ĵ��������仯ѧ����ʽ��4NH+5O2 4NO+6H2O
4NO+6H2O
��ŨH2SO4������ˮ�ԣ�Ҳ���ԺͰ�����Ӧ������B��ŨH2SO4������������ˮ�Ͷ���İ���
��4�����ڿ����IJ��Ϲ��룬����������ˮ��������ȫ��Ӧ�������ᣬ��ѧ����ʽΪ4NO2+O2+2H2O=4HNO3��3 NO2+H2O=2HNO3+NO
��5��a��ͨ����������þ��Dz�������NH3��NO
���㣺����10���������ƶϣ������ĺϳɣ��������ȡ����ѧƽ�ⳣ���ıȽϣ�ƽ��״̬���жϣ���ѧ����ʽ����д

����һ�������£���ѧ�����ô��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״������������ͼ��ʾ���Իش��������⣺
��1���úϳ�·�߶��ڻ��������ļ�ֵ���� ��
��2��15��20%���Ҵ�����HOCH2CH2NH2��ˮ��Һ���������ԣ������ϳ���·������CO2���ռ��������ӷ���ʽ��ʾ�Ҵ���ˮ��Һ�������Ե�ԭ�� ��
��3��CH3OH��H2��ȼ���ȷֱ�Ϊ����H����725.5 kJ/mol����H����285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��ȼú�����е�CO2ת��Ϊ���ѵķ�Ӧԭ��Ϊ��
2CO2(g) + 6H2(g)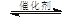 CH3OCH3(g) + 3H2O(g)
CH3OCH3(g) + 3H2O(g)
��֪һ��ѹǿ�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת���ʼ��±���
| Ͷ�ϱ�[n(H2) / n(CO2)] | 500 K | 600 K | 700 K | 800 K |
| 1.5 | 45% | 33% | 20% | 12% |
| 2.0 | 60% | 43% | 28% | 15% |
| 3.0 | 83% | 62% | 37% | 22% |
��4���÷�Ӧ���ʱ��H 0���ر��S 0�����������
��5���ü�����Ϊȼ�ϵ��ԭ�ϣ��ڼ��Խ����иõ�ظ����ĵ缫��Ӧʽ ��
��6������1.12 L��min-1����״������������õ����ͨ����ѣ��е�Ϊ-24.9 �棩���øõ�ص��500 mL 2 mol��L-1 CuSO4��Һ��ͨ��0.50 min�������Ͽ���������ͭ g��
(1)�ٸ�������ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ____________________________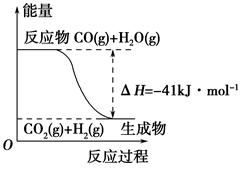
�ڸ�����ͼ��ʾ������ж�����˵������ȷ����______________��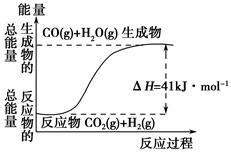
| A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)=CO2(g)��H2(g)����H��41 kJ��mol��1 |
| B���÷�ӦΪ���ȷ�Ӧ |
| C���÷�ӦΪ���ȷ�Ӧ |
| D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ��mol��1 |
(3)��ͼ��ij�¶��£�N2��H2��Ӧ�����������仯������ͼ���÷�Ӧ���Ȼ�ѧ����ʽΪ___________________________
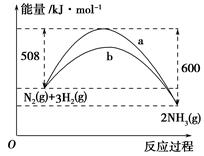
a��b�������߲��������ԭ��ܿ�����
_________________________________________________________________��
 ��
��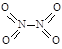 �� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��
�� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ��  NiO(OH)��MH
NiO(OH)��MH 2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ1��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺
2SO3��g���������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ1��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣺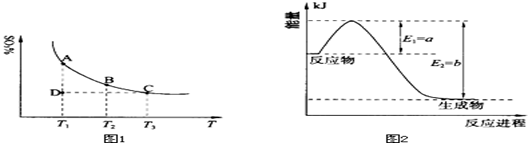
 CO(g) + 3H2(g) ��H=+206��2 kJ��mol��1
CO(g) + 3H2(g) ��H=+206��2 kJ��mol��1 CO(g) + 3H2(g) ��H>0
CO(g) + 3H2(g) ��H>0