��Ŀ����
7����1��CH3+��-CH3��������CH3-������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ����CD��A�����Ǿ��ɼ���ȥ��һ����ԭ������ B������̼ԭ�Ӿ���ȡsp2�ӻ�
C��CH3-��NH3��H3O+���ι��;�Ϊ������ D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ�����
��2��N2��O22+��Ϊ�ȵ����壬O22+�ĵ���ʽ�ɱ�ʾΪ
 ��1mol O22+�к��еĦм���ĿΪ2NA��
��1mol O22+�к��еĦм���ĿΪ2NA����3��X��Y�����л���ķ��ӽṹ�Ͳ��������������±����������������в������Ҫԭ����X�����γɷ����������Y�����γɷ��Ӽ������
| ���� | �ṹ��ʽ | ˮ���ܽ��/g��25�棩 | �۵�/�� | �е�/�� |
| X | 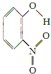 | 0.2 | 45 | 100 |
| Y | 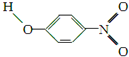 | 1.7 | 114 | 295 |
���� ��1��A������ȥ��һ����ԭ�ӵõ��������ܵõ�CH3+��CH3-��
B��CH3+��-CH3��CH3-�м۲���ӶԸ����ֱ���3��4��4�����ݼ۲���ӶԻ��������ж�Cԭ���ӻ����ͣ�
C��CH3-��NH3��H3O+������10�����ӣ���Ϊ�ȵ����壬�ṹ���ƣ�
D��CH3+�е�̼ԭ�Ӽ۲���ӶԸ�����3�����ݼ۲���ӶԻ��������ж�Cԭ���ӻ����ͣ�
��2���ȵ�����Ľṹ���ƣ����ݵ�������ʽ��дO22+����ʽ��ÿ��O22+����2���м���
��3�����Ӽ�����������ʵ��۷е����ߣ�����������������ʵ��۷е㽵�ͣ�
��� �⣺��1��A��������ӱ��CH3+��-CH3��CH3-ʱ��ʧȥ�ķֱ����⸺���ӡ���ԭ�Ӻ������ӣ��ռ乹��Ҳ������ԭ���ķ�����ͬ����A����
B��CH3+��-CH3��CH3-�м۲���ӶԸ����ֱ���3��4��4�����ݼ۲���ӶԻ�������֪��Cԭ���ӻ����ͷֱ���sp2��sp3��sp3����B����
C��CH3-��NH3��H3O+������8���۵��ӣ�4��ԭ�ӣ���Ϊ�ȵ����壬�ṹ���ƣ����������������νṹ�������⼸�������������Σ���C��ȷ��
D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ���ƽ�������νṹ������ԭ�Ӿ����棬��D��ȷ��
��ѡCD��
��2�����ݵȵ�����Ľṹ���ƣ�O22+�ĵ���ʽ  ����1��O22+����2���м�����1 mol O22+�У�����2NA�� �м���
����1��O22+����2�������1 mol O22+������2NA�� ���
�ʴ�Ϊ�� ��2NA��
��2NA��
��3��X�����γɷ�������������ʵ��۷е㽵�ͣ�Y�����γɷ��Ӽ���������ʵ��۷е����ߣ�
�ʴ�Ϊ��X�����γɷ����������Y�����γɷ��Ӽ������
���� ���⿼��ԭ���ӻ���ʽ�жϡ�������ȵ������֪ʶ�㣬���ؿ���������ۡ�������������ü۲���ӶԻ��������ж����ռ乹�ͼ�ԭ���ӻ���ʽ�жϣ�֪�����Ӱ���������ʲ�Ӱ�컯ѧ���ʣ���Ŀ�ѶȲ���

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�| A�� | H2O | B�� | Na2CO3��Һ | C�� | pH��ֽ | D�� | ʯ����ֽ |
| ���� �� | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� | ⑪ | ⑫ |
��2������ԭ�ӵĽṹʾ��ͼ����
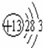 ��⑪
��⑪ ��
����3���ڢ١�⑫Ԫ���У���������ǿ��Ԫ����K���ǽ�������ǿ��Ԫ����F������õ�Ԫ����Ar��������Ԫ�ط��ţ�
��4��Ԫ�آ���Ԫ�آ���ȣ��ǽ����Խ�ǿ����Cl����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����b��
a�������¢ߵĵ��ʺ͢�ĵ���״̬��ͬ
b������⻯��Ȣߵ��⻯���ȶ�
c��һ�������¢ߺ͢�ĵ��ʶ���������������Һ��Ӧ
��5������������ԭ�Ӱ뾶����Ԫ�ظ���ͬ������ԭ�Ӱ뾶��С��Ԫ�أ�ϡ��������⣩�����γ����ӣ������ӻۣ������
��6��Ԫ�آ��ǹ��ɵؿǵ���ҪԪ��֮һ�������������ִ���ѧ��������Ʒ�Ļ���ԭ�ϣ���������Ļ�ѧʽΪSiO2���������к��еĻ�ѧ���ǹ��ۼ�������ӡ����ۡ���
��7��Ԫ�آ��Ƕ�ֲ����������ȱ�ٵ�Ԫ�أ��ǵ����ʵ���Ҫ�ɷ֣����⻯�ﳣ��������������⻯��ĵ���ʽΪ��
 ����һ�������£�����Ԫ�آٵĵ��ʺ������Ļ������ͨ��4L���ܱ������з�����Ӧ������Ӻ�������������ʵ���Ϊ1.4mol������Ԫ�آٵĵ��ʱ�ʾ�ķ�Ӧ����Ϊ0.35mol/��L•min����
����һ�������£�����Ԫ�آٵĵ��ʺ������Ļ������ͨ��4L���ܱ������з�����Ӧ������Ӻ�������������ʵ���Ϊ1.4mol������Ԫ�آٵĵ��ʱ�ʾ�ķ�Ӧ����Ϊ0.35mol/��L•min���� | A�� | ԭ�Ӱ뾶��A��B��C��D | B�� | ԭ��������b��a��c��d | ||
| C�� | �����ԣ�B��A���ǽ����ԣ�D��C | D�� | ���Ӱ뾶��D��C��A��B |
| A�� | ��ʵ��ⶨ�����ʵ��������ᡢ����ֱ�������NaOH��Һ��Ӧ�ų���������� | |
| B�� | �к��Ȳⶨʵ���п����û�����˿��������滷�β�������� | |
| C�� | �ü�ʽ�ζ�����ȡ0.10 mol•L-1��Na2CO3��Һ22.10 mL | |
| D�� | �ò�����պȡ��Һ�ε�ʪ��Ĺ㷶pH��ֽ�ϣ�����pH=3.6 |
| A�� | ��������MgCl2���� | B�� | ������ˮ | ||
| C�� | ��������Na2CO3���� | D�� | �����¶� |
| A�� | Br2�ڷ�Ӧ�б��������� | B�� | SO2�ڷ�Ӧ�б���ԭ | ||
| C�� | Br2�ڷ�Ӧ�еõ��� | D�� | 1mol�������ڷ�Ӧ�еõ�1mol���� |
| A�� | 25�� | B�� | 1.01��103Pa | C�� | 101kPa | D�� | 0�� |
 ʵ�����Ʊ��������ķ�Ӧԭ����ʵ��װ�����£�
ʵ�����Ʊ��������ķ�Ӧԭ����ʵ��װ�����£�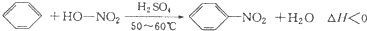
���ڵ���Ҫ����Ӧ�У����¶��Ըߵ�����»����ɼ�����������й����������
| ���� | �۵�/�� | �е�/�� | �ܶ�/g•cm-3 | �ܽ��� |
| �� | 5.5 | 80 | 0.88 | ����ˮ |
| ������ | 5.7 | 210.9 | 1.205 | ������ˮ |
| ��������� | 89 | 301 | 1.57 | ����ˮ |
| Ũ���� | 83 | 1.4 | ������ˮ | |
| Ũ���� | 338 | 1.84 | ������ˮ |
ȡ100mL�ձ�����20mLŨ������18mLŨ�������ƻ��Һ���������С�ļ���B�У���18mL��15.84g��������A�У��������µı�����μ�����ᣬ�ߵα߽��裬��Ͼ��ȣ���50��60���·�����Ӧ��ֱ����Ӧ������
����ӦҺ��ȴ�����º����Һ©���У�����������ˮ��5%NaOH��Һ��ˮϴ�ӣ��ֳ��IJ��������ˮCaCl2����������Ƭ�̣���ȥCaCl2�������������ռ�205��210����֣��õ���������18g���ش��������⣺
��1��װ��B�������Ƿ�Һ©����װ��C������������������
��2�����ƻ��Һʱ���ܷ�Ũ������뵽Ũ�����У�˵�����ɣ����ܣ��������Ž���
��3��Ϊ��ʹ��Ӧ��50�桫60���½��У����õķ�����ˮԡ���ȣ�
��4����ϴ�Ӳ����У��ڶ���ˮϴ��������ϴȥ������NaOH�����ɵ��Σ�
��5�������������У����������ķе����140�棬Ӧѡ�ÿ��������ܣ���ѡ��ֱ�������ܵ�ԭ��������ֱ��������ͨˮ��ȴʱ�����²���������ը�ѣ�
��6����ʵ�����õ���������������72%��