��Ŀ����
[��ѧ-ѡ��ѧ�뼼��]��ش��ȼ���������⣺
��1���������ռ��ǵ��ʳ��ˮʱ���չ̶��ı���k�������ȣ����ɵIJ�Ʒ��������k= ��Ҫ��������ʽ�ͽ������
��2��ԭ�ϴ����г�������ɳ��Ca2+��Mg2+��Fe3+��SO42-�����ʣ����뾫�ƺ���ܹ����ʹ�ã�����ʱ����������ˮ���˺�Ҫ������Լ��ֱ�Ϊ��Na2CO3����HCl�����ᣩ��BaCl2����3���Լ����ӵĺ���˳���� ������ţ�
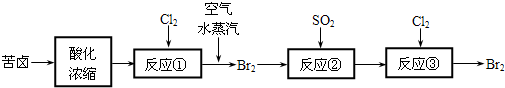
��3���ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խڣ��磩��30%���ϣ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ����

��ͼ��X��Y�ֱ��� �� ���ѧʽ���������Ƚ�ͼʾ������������������a%��b%�Ĵ�С ��
�ڷֱ�д��ȼ�ϵ��B�������������Ϸ����ĵ缫��Ӧ������ �������� ��
��������Ƶ���Ҫ�ڣ��磩��֮�����ڣ�д��2���� �� ��
���𰸡���������1�����ݵ�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ2NaCl+2H2O 2NaOH+H2��+Cl2�������ɵõ�k=
2NaOH+H2��+Cl2�������ɵõ�k= =
= ���Դ˼��㣻
���Դ˼��㣻
��2��ץס��������Ҫ���ڳ�̼�������ǰ���ɵõ�˳���ϵ���ۢ٢ڣ�
��3������ͻ�ƿ�����Bȼ�ϵ����ߣ�ͨ����һ��Ϊ��������ԭ��Ӧ������ô��߱�ȻͨH2������Y��ΪH2����ת��������ݵ�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ��֪Ψһδ֪��X��ȻΪCl2��A�е�NaOH����ȼ�ϵ�������ٳ���������O2+4e-+2H2O=4OH-��֪NaOHŨ������
����⣺��1����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ2NaCl+2H2O 2NaOH+H2��+Cl2������������k=
2NaOH+H2��+Cl2������������k=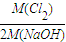 =
=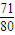 =1��1.13��0.89��
=1��1.13��0.89��
�ʴ�Ϊ��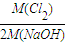 =
=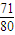 =1��1.13��0.89��
=1��1.13��0.89��
��2��Ӧ�ȼ������BaCl2����ȥSO42-��Ȼ��ӹ���Na2CO3���ɳ�ȥCa2+��Mg2+��Fe3+�ȣ����˺��������ɳ�ȥNa2CO3���ʴ�Ϊ���ۢ٢ڣ�
��3����ͨ����һ��Ϊ��������ԭ��Ӧ������ô��߱�ȻͨH2������Y��ΪH2����������ӦΪ����������Cl2������ȼ�ϵ����������O2+4e-+2H2O=4OH-��ȼ�ϵ���е�����Ĥֻ����������ͨ������ȼ�ϵ�������������õ����Ӳ���OH-�����Է�Ӧ���������Ƶ�Ũ�����ߣ���a%С��b%����֪NaOHŨ������
�ʴ�Ϊ��Cl2��H2��a%С��b%��
��ȼ�ϵ�������������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪO2+4e-+2H2O=4OH-����������ʧ���ӷ���������Ӧ���缫��ӦʽΪH2-2e-+2OH-=2H2O��
�ʴ�Ϊ��O2+4e-+2H2O=4OH-��H2-2e-+2OH-=2H2O��
�۾�װ���ص㼰��Ӧ���̿�֪��������Ƶ��ŵ���ȼ�ϵ�ؿ��Բ���������ĵĵ��ܣ���߲�����Һ��Ũ�ȣ������ܺĵȣ�
�ʴ�Ϊ��ȼ�ϵ�ؿ��Բ���������ĵĵ��ܣ���߲�����Һ��Ũ�ȣ�
����������ǰ�벿�ֻ��������ȼ�յ����֣�����벿��ԭ��ء��������֪ʶ������ں���һ��ʹ������ֳ��˽ϺõĴ��⣬ѧϰ��Ҫ���ȼҵ�����̼��̲ĵ�ⱥ��ʳ��ˮʵ���漰���ĸ���֪ʶ��Ҫ��˳����ϸ���籥��ʳ��ˮ�ľ��ơ����ʱ�����缫������顢���ʼ��㡢�缫��Ӧʽ����ⷴӦ�Ļ�ѧ����ʽ��д��֪ʶҪ�������У�����һ��������������
 2NaOH+H2��+Cl2�������ɵõ�k=
2NaOH+H2��+Cl2�������ɵõ�k= =
= ���Դ˼��㣻
���Դ˼��㣻��2��ץס��������Ҫ���ڳ�̼�������ǰ���ɵõ�˳���ϵ���ۢ٢ڣ�
��3������ͻ�ƿ�����Bȼ�ϵ����ߣ�ͨ����һ��Ϊ��������ԭ��Ӧ������ô��߱�ȻͨH2������Y��ΪH2����ת��������ݵ�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ��֪Ψһδ֪��X��ȻΪCl2��A�е�NaOH����ȼ�ϵ�������ٳ���������O2+4e-+2H2O=4OH-��֪NaOHŨ������
����⣺��1����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ2NaCl+2H2O
 2NaOH+H2��+Cl2������������k=
2NaOH+H2��+Cl2������������k=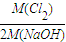 =
=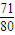 =1��1.13��0.89��
=1��1.13��0.89���ʴ�Ϊ��
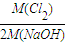 =
=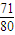 =1��1.13��0.89��
=1��1.13��0.89����2��Ӧ�ȼ������BaCl2����ȥSO42-��Ȼ��ӹ���Na2CO3���ɳ�ȥCa2+��Mg2+��Fe3+�ȣ����˺��������ɳ�ȥNa2CO3���ʴ�Ϊ���ۢ٢ڣ�
��3����ͨ����һ��Ϊ��������ԭ��Ӧ������ô��߱�ȻͨH2������Y��ΪH2����������ӦΪ����������Cl2������ȼ�ϵ����������O2+4e-+2H2O=4OH-��ȼ�ϵ���е�����Ĥֻ����������ͨ������ȼ�ϵ�������������õ����Ӳ���OH-�����Է�Ӧ���������Ƶ�Ũ�����ߣ���a%С��b%����֪NaOHŨ������
�ʴ�Ϊ��Cl2��H2��a%С��b%��
��ȼ�ϵ�������������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪO2+4e-+2H2O=4OH-����������ʧ���ӷ���������Ӧ���缫��ӦʽΪH2-2e-+2OH-=2H2O��
�ʴ�Ϊ��O2+4e-+2H2O=4OH-��H2-2e-+2OH-=2H2O��
�۾�װ���ص㼰��Ӧ���̿�֪��������Ƶ��ŵ���ȼ�ϵ�ؿ��Բ���������ĵĵ��ܣ���߲�����Һ��Ũ�ȣ������ܺĵȣ�
�ʴ�Ϊ��ȼ�ϵ�ؿ��Բ���������ĵĵ��ܣ���߲�����Һ��Ũ�ȣ�
����������ǰ�벿�ֻ��������ȼ�յ����֣�����벿��ԭ��ء��������֪ʶ������ں���һ��ʹ������ֳ��˽ϺõĴ��⣬ѧϰ��Ҫ���ȼҵ�����̼��̲ĵ�ⱥ��ʳ��ˮʵ���漰���ĸ���֪ʶ��Ҫ��˳����ϸ���籥��ʳ��ˮ�ľ��ơ����ʱ�����缫������顢���ʼ��㡢�缫��Ӧʽ����ⷴӦ�Ļ�ѧ����ʽ��д��֪ʶҪ�������У�����һ��������������

��ϰ��ϵ�д�
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
�����Ŀ
����ѧ--ѡ��ѧ�뼼����
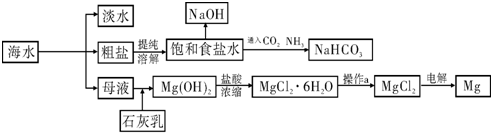
��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
��ش��������⣺
��1�����о�һ�ֺ�ˮ�����ķ��� ����
��2����ҵ�ϳ������ӽ���Ĥ��������NaOH���������д���ͨ�����ӽ���Ĥ�������� ��NaOH�ڵ��۵� �����ɣ��ɱ���ʳ��ˮ��ȡNaOH�Ļ�ѧ����ʽΪ ��
��3�������Ƽ��������ʳ��ˮ��ͨ��CO2��NH3�Ƶ�NaHCO3����ͨ��
���ѧʽ���������� ������NaHCO3�Ƶô����ѧ����ʽΪ ��
��4��þ��һ����;�ܹ㷺�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ����֪�й����ʵ��۷е��������£�
��ϱ������ݺ�ʵ���������˵������ҵ������þ���õ��MgCl2�����ǵ��MgO������ ��
��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��

��ش��������⣺
��1�����о�һ�ֺ�ˮ�����ķ���
��2����ҵ�ϳ������ӽ���Ĥ��������NaOH���������д���ͨ�����ӽ���Ĥ��������
��3�������Ƽ��������ʳ��ˮ��ͨ��CO2��NH3�Ƶ�NaHCO3����ͨ��
���ѧʽ����������
��4��þ��һ����;�ܹ㷺�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ����֪�й����ʵ��۷е��������£�
| MgO | MgCl2 | |
| �۵�/�� | 2852 | 714 |
| �е�/�� | 3600 | 1412 |
 2NH3��+CaCl2+2H2O
2NH3��+CaCl2+2H2O Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O