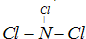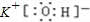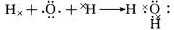��Ŀ����
����A��B��C��D��E��������Ԫ�أ����ǵĺ˵������������
��A�ĺ˵����С��10�����γ���̬�⻯��������ڳ�����ΪҺ�壬
�� A��C����Ԫ�ؿ��γɻ�����C2A3���û����������ǿ�ᣬ������ǿ�
��B����������B+��C3+������ͬ�ĵ��Ӳ�ṹ��
�� B��E���γɻ�����BE��
��BE��Һ��ͨ��Cl2�ӵ��۱���ɫ��
�������ڱ��У�D����B����һ�����ڣ���������D��������ˮ��Ӧ�ų���������D���������ɵ��������ʵ���֮��Ϊ2��1��
(1) д������Ԫ�ص����ƣ�A _____________�� E______________ ��
(2) д������Ԫ�ص�ԭ��������B__________ �� D_____________ ��
(3) д�����з����ķ�Ӧ�Ļ�ѧ����ʽ��_____________________________
(4)C2A3��NaOH��Һ��Ӧ�����ӷ���ʽΪ�� _____________________________
(5) �����ʵ��Ƚ�B��D�Ľ�����ǿ����
��A�ĺ˵����С��10�����γ���̬�⻯��������ڳ�����ΪҺ�壬
�� A��C����Ԫ�ؿ��γɻ�����C2A3���û����������ǿ�ᣬ������ǿ�
��B����������B+��C3+������ͬ�ĵ��Ӳ�ṹ��
�� B��E���γɻ�����BE��
��BE��Һ��ͨ��Cl2�ӵ��۱���ɫ��
�������ڱ��У�D����B����һ�����ڣ���������D��������ˮ��Ӧ�ų���������D���������ɵ��������ʵ���֮��Ϊ2��1��
(1) д������Ԫ�ص����ƣ�A _____________�� E______________ ��
(2) д������Ԫ�ص�ԭ��������B__________ �� D_____________ ��
(3) д�����з����ķ�Ӧ�Ļ�ѧ����ʽ��_____________________________
(4)C2A3��NaOH��Һ��Ӧ�����ӷ���ʽΪ�� _____________________________
(5) �����ʵ��Ƚ�B��D�Ľ�����ǿ����

(1)A:�� ��E:��
(2)B��11��D:19
(3) 2NaI+Cl2==2NaCl+I2
(4)Al2O3+2OH-==2AlO2-+H2O
(5) ʵ����ƣ���ȡһС��Na��K������ˮ�У��۲���ˮ��Ӧ�ľ��ҳ̶�
����K��ˮ��Ӧ��Na��ˮ��Ӧ����
�����ԣ�K > Na
(2)B��11��D:19
(3) 2NaI+Cl2==2NaCl+I2
(4)Al2O3+2OH-==2AlO2-+H2O
(5) ʵ����ƣ���ȡһС��Na��K������ˮ�У��۲���ˮ��Ӧ�ľ��ҳ̶�
����K��ˮ��Ӧ��Na��ˮ��Ӧ����
�����ԣ�K > Na

��ϰ��ϵ�д�
�����Ŀ
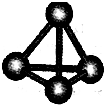 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��