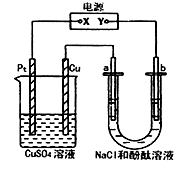
��Ŀ����
��7�֣���֪20��ʱNaCl���ܽ��Ϊ36 g��ij�ȼʹ�õ��豸�������ӽ���Ĥ���ۣ���һ��������м���һ����20��ʱ����NaCl��Һ����90%��NaCl���ʱ�������ռ���11. 2 m3���壨������ɱ�״��������ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2��ȡ��������Һ��ϵ��ʵ�飬���н����д������ ��������ĸ��
��3������ԭ����NaCl��Һ������ kg����ȷ��0.1kg����
��4�������90%��NaCl����������ɼ���ȼ�ϵ���ṩ��������������� m3��
������ĵ缫��Ӧʽ��CH4+10OH��-8e�� = CO32��+7H2O
�����ȼ�ϵ�ص�����������Ϊ90%������ɱ�״������ȷ��0.1 m3��
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2��ȡ��������Һ��ϵ��ʵ�飬���н����д������ ��������ĸ��
| A���μӷ�̪�Լ����ȱ�����ɫ | B���μ���������Һ���а�ɫ�������� |
| C���μ�С�մ���Һ�������ݲ��� | D������ɫ��Ӧʵ��ʻ�ɫ |
��4�������90%��NaCl����������ɼ���ȼ�ϵ���ṩ��������������� m3��
������ĵ缫��Ӧʽ��CH4+10OH��-8e�� = CO32��+7H2O
�����ȼ�ϵ�ص�����������Ϊ90%������ɱ�״������ȷ��0.1 m3��
��1��2Cl��+2H2O 2OH��+Cl2��+H2����2�֣�
2OH��+Cl2��+H2����2�֣�
��2��A��2�֣� ��3��245.6 kg��1�֣� ��4��3.1m3��1�֣�
 2OH��+Cl2��+H2����2�֣�
2OH��+Cl2��+H2����2�֣���2��A��2�֣� ��3��245.6 kg��1�֣� ��4��3.1m3��1�֣�
��1�����Ե缫����Ȼ�����Һ�������������ӷŵ磬�����������ӷŵ磬�ܷ�ӦʽΪ2Cl��+2H2O 2OH��+Cl2��+H2����
2OH��+Cl2��+H2����
��2�������������ӷŵ磬������������������ˮ���ɴ����ᣬ�����ԣ�����ѡ��A�Ǵ���ģ������ܱ��ɫ������ѡ�����ȷ�ġ�
��3����ԭ����NaCl��Һ��������x�������ռ������������������ʵ�����500mol�����Ա������Ȼ�����1000mol��������58500g�������� �����x��245.6kg��
�����x��245.6kg��
��4����Ӧ��ת�Ƶ�����1000mol��������Ҫ������1000��8��0.9��138.9mol�������Լ��3.1m3��
 2OH��+Cl2��+H2����
2OH��+Cl2��+H2������2�������������ӷŵ磬������������������ˮ���ɴ����ᣬ�����ԣ�����ѡ��A�Ǵ���ģ������ܱ��ɫ������ѡ�����ȷ�ġ�
��3����ԭ����NaCl��Һ��������x�������ռ������������������ʵ�����500mol�����Ա������Ȼ�����1000mol��������58500g��������
 �����x��245.6kg��
�����x��245.6kg����4����Ӧ��ת�Ƶ�����1000mol��������Ҫ������1000��8��0.9��138.9mol�������Լ��3.1m3��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ���������ӣ����м���˳��������� ����
���������ӣ����м���˳��������� ����