��Ŀ����
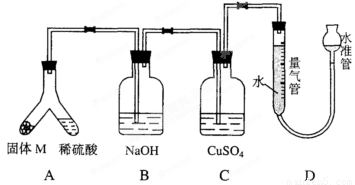
ij������ȤС��Ϊ��̽���������ڸ��������������·�Ӧ���ù���M�ijɷ֡��������ͼ1-5-29װ�á���бAʹϡ����(����)�����M��ַ�Ӧ������Ӧֹͣ��Bװ�����أ�Cװ������Һ�ޱ仯����Ӧ�������������������Ϊ VmL(������ɱ�״��)��������ʵ����ʵ��֪��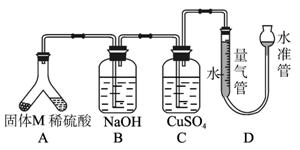
ͼ1-5-29
(1)�ٹ���M��һ���е�������_____________ (�ѧʽ)
������_____________________________________________________________��
������һ�����ʵ���������ȷ��Ϊ__________g(�ô���ʽ��ʾ)��
(2)Bװ�õ�������____________________��
д��Bװ���з�Ӧ�����ӷ���ʽ________________________________________________��
(3)Cװ�õ�������______________________________�����ʵ����û��Bװ�ã���Cװ���в�����������______________________________��
(4)ϡ�������M��Ӧ����Һ�л���������ɫ���壬�ù�����__________��Ҫ������ù��塣��ʵ������У����ձ����Ҫ�õ��IJ���������______________________________��
(5)ͨ����һ��ʵ�飬��ù���M�и��ֳɷֵ�����֮��С�ڷ�Ӧǰ���ۺ���۵�����֮�͡��������������ԭ�������__________________________________________________��
a.M����δ��Ӧ��������
b.�ⶨ�������ʱˮ�ܵ�ˮ����������ܵ�ˮ��
c.A�����з�Ӧ���ɵ�����
d.�������Dװ��ǰδ��Ũ�������
����������ͨ��NaOH��Һʱ��NaOH��Һ�������ӣ�˵��Fe��S�ڸ���������������
��Ӧ��������һ������FeS��H2S����ͨ��NaOH����ͨ��CuSO4��Һ�����Ա仯��˵��H2S��ͨ��NaOH��Һʱ��ȫ�������գ�����H2S�������CuSO4���ò�����ɫ����CuS��
����������������ռ���������һ����H2����M��һ������Fe���ʡ��ָ��ݵ��ӵ�ʧ�غ���n(Fe)��2=n(H2)��2=![]() ��2����m(Fe)=
��2����m(Fe)=![]() ��56 g��mol-1=
��56 g��mol-1=![]() g����H2SO4��Ӧ����Һ�л������е���ɫ�����ʣ����ù��˵ķ��������ȥ�����貣���������ձ���©���Ͳ�������M�и��ɷݵ�����֮��С�����ۺ���۵���������ԭ������Dz�����H2SO4��Ӧ����������δȫ�����գ�Ҳ���������ڲⶨ���������ȷ��ɵģ���ˮ��Һ�����������Һ��ȡ�
g����H2SO4��Ӧ����Һ�л������е���ɫ�����ʣ����ù��˵ķ��������ȥ�����貣���������ձ���©���Ͳ�������M�и��ɷݵ�����֮��С�����ۺ���۵���������ԭ������Dz�����H2SO4��Ӧ����������δȫ�����գ�Ҳ���������ڲⶨ���������ȷ��ɵģ���ˮ��Һ�����������Һ��ȡ�
�𰸣�(1)��FeS��Fe
Bװ������H2S���أ�����FeS V mL��������Fe��H2SO4��Ӧ������H2������Fe��
��V/400
(2)ϴ��ƿ H2S+2 OH-![]() S2-+2H2O(��H2S+ OH-
S2-+2H2O(��H2S+ OH-![]() HS-+H2O)
HS-+H2O)
(3)����H2S�Ƿ�������ȫ �к�ɫ����
(4)��(��S) ©���������� (5)b c