��Ŀ����
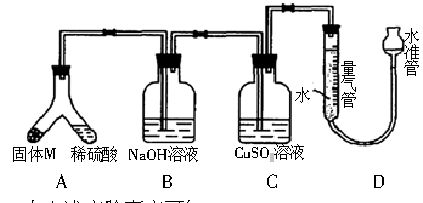
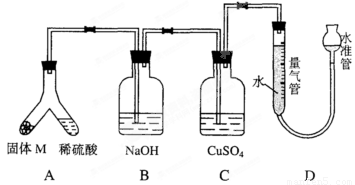
ij������ȤС��Ϊ��̽���������ڸ��������������·�Ӧ���ù���M�ijɷ֣����������ͼװ�á���бAʹϡ����(����)�����M��ַ�Ӧ������Ӧֹͣ��Bװ�����أ�Cװ������Һ�ޱ仯����Ӧ�������������������ΪV mL(������ɱ�״��)��������ʵ����ʵ��֪��
(1)�ٹ���M��һ���е�������__________(�ѧʽ)��
��M��һ�����ʵ���������ȷ��Ϊ__________g(�ô���ʽ��ʾ)��
(2)д��Bװ���з�Ӧ�����ӷ���ʽ____________________��
(3)Cװ�õ�������____________________��
(4)ϡ�������M��Ӧ����Һ�л���������ɫ���壬Ҫ������ù��壬��ʵ������У����ձ����Ҫ�õ��IJ���������____________________��
(5)ͨ����һ��ʵ�飬��ù���M�и��ֳɷֵ�����֮��С�ڷ�Ӧǰ���ۺ���۵�����֮�ͣ��������������ԭ�������____________________��
a.M����δ��Ӧ��������
b.�ⶨ�������ʱˮ�ܵ�ˮ����������ܵ�ˮ��
c.A�����з�Ӧ���ɵ�����
d.�������Dװ��ǰδ��Ũ�������
(6)Dװ�����ڶ�ȡ��������ʱ������Ҫʹ����������¶���ȴ�����£���Ӧע���������____________________________________________________________________��
(1)��FeS��Fe ��V/400
(2)2OH-+H2S![]() 2H2O+S2-
2H2O+S2-
(3)֤��H2S�ѱ���ȫ����
(4)©����������
(5)bc
(6)ͨ���ƶ�ˮ�ܣ�ʹˮ�ܺ��������ڵ�Һ����ͬһ�߶�
��������������۸���������Ӧ�ù���M������M��ֻ����Fe��S����Ԫ�أ���Bװ�����أ�˵������M��ϡ���ᷴӦ������H2S���壬Cװ����Һ�ޱ仯��˵��H2S��ȫ����NaOH��Һ���գ������ӷ���ʽΪ2OH-+H2S![]() S2-+2H2O�����Dװ���ռ���V mL���壬˵������M��ϡ���ᷴӦ������H2���ɴ˿���˵������M��һ������FeS��Fe.
S2-+2H2O�����Dװ���ռ���V mL���壬˵������M��ϡ���ᷴӦ������H2���ɴ˿���˵������M��һ������FeS��Fe.
Fe+2H+![]() Fe2++H2��
Fe2++H2��
M V��10
m=![]() g
g
ϡ�������M��Ӧ����Һ�л���������ɫ���壬Ҫ������ù��壬���˼��ɣ����ձ��⣬����Ҫ�IJ���������©���Ͳ�����.

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�