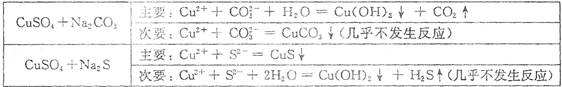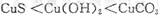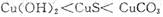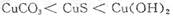��Ŀ����
�� 4 mol A ����� 2 mol B ������ 2 L �������л�ϲ���һ�������·������·�Ӧ:2A��������B��������2C������������ 2 s���룩���� C ��Ũ��Ϊ 0.6 mol��L��1 ���������м���˵����
�������� A ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.3 mol��L��1��s��1
�������� B ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.6 mol��L��1��s��1
�� 2 s ʱ���� A ��ת����Ϊ70��
�� 2 s ʱ���� B ��Ũ��Ϊ 0.7 mol��L��1������ȷ���ǣ� ��
�������� A ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.3 mol��L��1��s��1
�������� B ��ʾ�ķ�Ӧ��ƽ������Ϊ 0.6 mol��L��1��s��1
�� 2 s ʱ���� A ��ת����Ϊ70��
�� 2 s ʱ���� B ��Ũ��Ϊ 0.7 mol��L��1������ȷ���ǣ� ��
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
B
��������� 2A��������B��������2C������
��ʼŨ�ȣ�mol/L�� 2 1 0
ת��Ũ�ȣ�mol/L�� 0.6 0.3 0.6
2s��Ũ�ȣ�mol/L�� 1.4 0.7 0.6
���������� A ��ʾ�ķ�Ӧ��ƽ������Ϊ0.6mol/L��2s�� 0.3 mol��L��1��s��1
������ B ��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol/L��2s�� 0.15mol��L��1��s��1
2 s ʱ���� A ��ת����Ϊ0.6��2��30��
������ȷ�Ĵ�ѡB��
�������ڽ��п��淴Ӧ���йؼ���ʱ��һ����á�����ʽ�����У����ֱ��г���ʼ����ת������ƽ������ij��̵�����Ȼ��������֪������ʽ���㼴�ɡ�

��ϰ��ϵ�д�
�����Ŀ
 CH3OH��g����
CH3OH��g����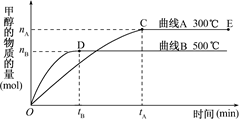
 cC��g��+ dD��g��������ͼ�ش�
cC��g��+ dD��g��������ͼ�ش�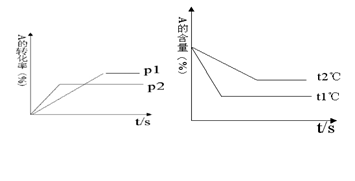
 2C(g)�ڶ����ܱ������дﵽƽ��ı�־���ǣ���C������������C�ķֽ�������ȣ��ڵ�λʱ����amol A���ɣ�ͬʱ����3amolB����A��B��C��Ũ�Ȳ��ٱ仯���ܻ���������ѹǿ���ٱ仯���ݻ�������ƽ��Ħ���������ٱ仯������A��B��C�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ1:3:2����A��B��C�ķ�����Ŀ��Ϊ1:3:2������������ܶȲ��ٱ仯
2C(g)�ڶ����ܱ������дﵽƽ��ı�־���ǣ���C������������C�ķֽ�������ȣ��ڵ�λʱ����amol A���ɣ�ͬʱ����3amolB����A��B��C��Ũ�Ȳ��ٱ仯���ܻ���������ѹǿ���ٱ仯���ݻ�������ƽ��Ħ���������ٱ仯������A��B��C�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ1:3:2����A��B��C�ķ�����Ŀ��Ϊ1:3:2������������ܶȲ��ٱ仯 SiF4(g)+ 2H2O(g) ��H =" ��148.9" kJ/mol
SiF4(g)+ 2H2O(g) ��H =" ��148.9" kJ/mol
 3C(g)+D(g)��Ӱ�죬���ҵ�ѹǿ��
3C(g)+D(g)��Ӱ�죬���ҵ�ѹǿ�� 3C(g)����2 s��ﵽƽ�⣬���C�����Ũ��
3C(g)����2 s��ﵽƽ�⣬���C�����Ũ�� 2C (g) ������2s����C��Ũ��Ϊ 0.6mol/L ������˵����ȷ����
2C (g) ������2s����C��Ũ��Ϊ 0.6mol/L ������˵����ȷ����