��Ŀ����
����Ŀ��˫��ˮ(���������ˮ��Һ)�ڹ�ҵ�������ճ�������Ӧ�ù㷺��
(1)˫��ˮ�������˿���������һ���������˹��������_________(���������Ļ�ѧ����)��
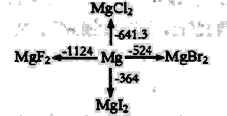
(2)����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N2H4)��ǿ������Һ̬�������⡣��֪0.4 molҺ̬����������Һ̬�������ⷴӦ�����ɵ�����ˮ���������ų�256.652kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
(3)˫��ˮ�ܽ����Է�ˮ�е�CN��ת����̼���κ�һ�ֶԻ�������Ⱦ�����壬CN���ĵ���ʽΪ________��д���÷�Ӧ�����ӷ���ʽ________��
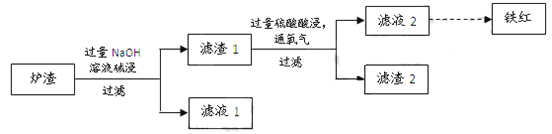
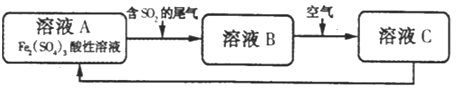
(4)��ͼ�ǹ�ҵ���Ʊ�������������ķ�����д��ʵ�ʷ�����Ӧ���ܷ���ʽ_______��
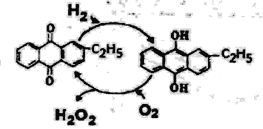
(5)��ͼ��һ���õ��ԭ�����Ʊ�H2O2�����ò�����H2O2�����ϰ�ˮ��װ�á�
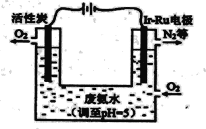
��Ir��Ru���Ե缫����O2����H2O2����缫��Ӧʽ��_______��
�ڴ����ϰ�ˮ������(��NH3��)��������34g�������ϵ�·��ת�Ƶ�����Ϊ__________mol��
���𰸡�ǿ������ N2H4(l)+2H2O2��l��= N2��g��+4H2O��g�� ��H= -641.63kJ/mol ![]() 5H2O2+2CN��+2OH����N2��+2CO32��+6H2O H2+O2
5H2O2+2CN��+2OH����N2��+2CO32��+6H2O H2+O2 H2O2 O2+2H++2e-=H2O2 6mol��
H2O2 O2+2H++2e-=H2O2 6mol��
��������
��1��˫��ˮ�������˿���������һ���������˹��������ǿ�����ԡ�
��2����Ӧ����ʽΪ��N2H4+2H2O2�TN2+4H2O��0.4molҺ̬�·ų�256.652KJ����������1molҺ̬�·ų�������Ϊ265.652KJ��0.4=641.63kJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4��g��+2H2O2��l���TN2��g��+4H2O��g����H=-641.63kJ��mol��1��
��3��CN���ĵ���ʽΪ��![]() ��˫��ˮ�ܽ����Է�ˮ�е�CN��ת����̼���κ�һ�ֶԻ�������Ⱦ�����壬���������������ﵪ�����÷�Ӧ�����ӷ���ʽ��5H2O2+2CN��+2OH����N2��+2CO32��+6H2O��
��˫��ˮ�ܽ����Է�ˮ�е�CN��ת����̼���κ�һ�ֶԻ�������Ⱦ�����壬���������������ﵪ�����÷�Ӧ�����ӷ���ʽ��5H2O2+2CN��+2OH����N2��+2CO32��+6H2O��
��4����ҵ���Ʊ�������������ķ�������ͼ�����ڴ��������£�������������Ӧ���ɹ������⣬ʵ�ʷ�����Ӧ���ܷ���ʽH2+O2 H2O2��
H2O2��
��5����Ir��Ru���Ե缫����O2����H2O2�������õ��ӷ�����ԭ��Ӧ����缫��Ӧʽ�� O2+2H++2e-=H2O2 ��
��4NH3+3O2=2N2+6H2O�У������еĵ�Ԫ�ش�-3�۱�Ϊ�����е�0�ۣ�4mol����ת��12mol���ӣ�����NH3�����ʵ���Ϊ1mol������Ϊ17g��ת��3mol���ӣ��ִ����ϰ�ˮ������(��NH3��)��������34g�������ϵ�·��ת�Ƶ�����Ϊ6mol��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���������ƣ���ѧʽΪ NaNO2����һ�ֳ��õķ��������ش���������:
��1��NaNO2 �� N Ԫ�صĻ��ϼ�Ϊ_________.
��2������������ 320��C ʱ�ֽܷ���������ƹ��塢һ��������һ�ֳ�������ȼ�����塣�÷�Ӧ�Ļ�ѧ����ʽ_________________��
��3���ҹ��涨���ȳ��������������ӱ�Ϊÿǧ��ʳƷ���������� 150 ���ˣ��Դ˼��㣬200g 15��������������Һ���ٿ������������ȳ�______ǧ�ˡ�
��4�������������£�NaNO2�밴���ʵ��� 1:1 ǡ����ȫ��Ӧ����I��������Ϊ I2ʱ�������к���������Ϊ________���ѧʽ����
��5����ҵ��ˮ�е� NaNO2 �������۳�ȥ����֪����ϵ�а��� AI��NaAlO2��NaNO2��NaOH��NH3��H2O �������ʡ��÷�Ӧ�Ļ�ѧ����ʽΪ____________��
��6��ijͬѧ���ʵ��Թ�ҵ��Ʒ�� NaNO2 �ĺ������вⶨ����ȡ������Ʒ 2g����ȫ�ܽ����Ƴ���Һ 100mL ȡ�� 25mL ��Һ�� 0.100 mol/L ���� KMnO4 ��Һ���еζ������ʲ��� KMnO4 ��Ӧ����ʵ�������������±���ʾ:
����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ���/mL | 20.70 | 20.02 | 20.00 | 19.98 |
����Ʒ���������Ƶ���������Ϊ_________.����֪:5NO2-+2MnO4-+6H+ = 5NO3-+2Mn2++3H2O��