��Ŀ����
����Ŀ�����ݻ�ѧ��Ӧ�е������仯��ϵ�ش��������⣺
��1���Ͽ�1molH-H����1molN-H����1molN��N���ֱ���Ҫ���յ�����Ϊ436kJ��391kJ��946kJ����
��1molN2����NH3��______�����������������ų���������______kJ��
��1molH2����NH3��______�����������������ų���������______kJ��
��2������ɴ�������(N2H4)������Դ����֪1molҺ̬�º�������Һ̬�������ⷴӦ���ɵ�����ˮ����ʱ�ų�641.6kJ��������ѧ����ʽ���£�N2H4��2H2O2===N2����4H2O��
���÷�Ӧ������ (��������ԭ������
���÷�Ӧ�ķ�Ӧ�������� ������ڻ���ڣ����������������
��������£�Һ̬��ȼ������0.5molN2ʱ�ų�������Ϊ kJ��
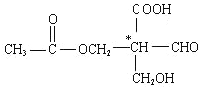
����(N2H4)�����д��ڵĻ�ѧ���� ��
��������1���ٷų���92���ڷų���30.7
��2������ԭ ������ ��320.8 �����Լ��ͷǼ��Լ�
��������
�����������1���Ͽ�1molH-H����1molN-H����1molN��N���ֱ���Ҫ���յ�����Ϊ436kJ��391kJ��946kJ��������2mol�����ķ�Ӧ����H��3��436kJ/mol��946kJ/mol��2��3��391kJ/mol����92kJ/mol����
��1molN2����NH3��ų�����92kJ��
��1molH2����NH3��ų�����![]() ��30.7kJ��
��30.7kJ��
��2�����÷�Ӧ��NԪ���Ļ��ϼ۴ӣ�2�����ߵ�0����ʧȥ���ӱ����������������ԭ����
���÷�Ӧ�����ȷ�Ӧ������Ӧ���������������������������
��������£�Һ̬��ȼ������0.5molN2ʱ�ų�������Ϊ641.6kJ��0.5��320.8kJ��
���·���������ʽΪ![]() �������д��ڵĻ�ѧ�������Լ����Ǽ��Լ���
�������д��ڵĻ�ѧ�������Լ����Ǽ��Լ���

 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�����Ŀ��������(CH3OCH3)��һ��������Դ�����ö�����̼�ϳ�����Դ�ѳ�Ϊ������ѧ���о������ſ��⡣
��1�����ֹ��ۼ��ļ��������ʾ��
��ѧ�� | C=O | H-H | C-H | C-O | H-O |
����/kJ��mol-1 | 803 | 436 | 414 | 326 | 464 |
2CO2(g)+6H2(g)=CH3OCH3(g)+3H2O(g) ![]() _______________��
_______________��
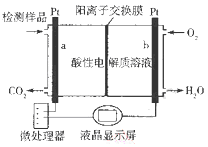
��2�����˹�������á�����������Ŀ���ü���ģ������Ĺ�����ã�����̫���⽫H2O��CO2ֱ�Ӻϳ�ȼ�Ϻͻ���ԭ�ϡ�������Աģ�������ã������ͼ����ʾװ���Ʊ������ѡ�����ת����ʽΪ̫����![]() ����
����![]() ��ѧ�ܡ�
��ѧ�ܡ�
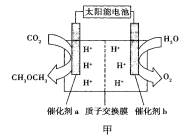
������b�ĵ缫������_________________��
�����ͷ�11.2L��������״���£�����___________mol H+___________�������ӵ�Ǩ�Ʒ���
������a�ϵĵ缫��ӦʽΪ______________��
��3����ʢ�ٴ�ѧ���о���Ա�о���һ�ַ�������ʵ��ˮ������ʱCO2�����ŷţ������ԭ����ͼ����ʾ��
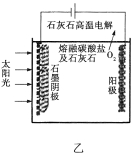
������������������ת����ʽ��__________________��
����ⷴӦ���¶�С��900![]() ʱ���У�̼��Ʒֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ_______________�������ĵ缫��ӦʽΪ______________��
ʱ���У�̼��Ʒֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ_______________�������ĵ缫��ӦʽΪ______________��