��Ŀ����
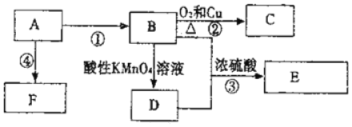
����Ŀ��������(CH3OCH3)��һ��������Դ�����ö�����̼�ϳ�����Դ�ѳ�Ϊ������ѧ���о������ſ��⡣
��1�����ֹ��ۼ��ļ��������ʾ��
��ѧ�� | C=O | H-H | C-H | C-O | H-O |
����/kJ��mol-1 | 803 | 436 | 414 | 326 | 464 |
2CO2(g)+6H2(g)=CH3OCH3(g)+3H2O(g) ![]() _______________��
_______________��
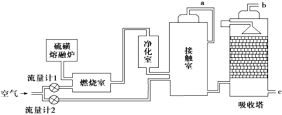
��2�����˹�������á�����������Ŀ���ü���ģ������Ĺ�����ã�����̫���⽫H2O��CO2ֱ�Ӻϳ�ȼ�Ϻͻ���ԭ�ϡ�������Աģ�������ã������ͼ����ʾװ���Ʊ������ѡ�����ת����ʽΪ̫����![]() ����
����![]() ��ѧ�ܡ�
��ѧ�ܡ�
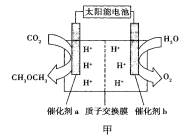
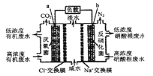
������b�ĵ缫������_________________��
�����ͷ�11.2L��������״���£�����___________mol H+___________�������ӵ�Ǩ�Ʒ���
������a�ϵĵ缫��ӦʽΪ______________��
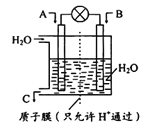
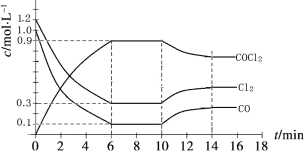
��3����ʢ�ٴ�ѧ���о���Ա�о���һ�ַ�������ʵ��ˮ������ʱCO2�����ŷţ������ԭ����ͼ����ʾ��
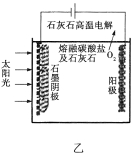
������������������ת����ʽ��__________________��
����ⷴӦ���¶�С��900![]() ʱ���У�̼��Ʒֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ_______________�������ĵ缫��ӦʽΪ______________��
ʱ���У�̼��Ʒֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ_______________�������ĵ缫��ӦʽΪ______________��
���𰸡���1��-92KJ![]() mol��1
mol��1
��2����������2 ��������Ҳ��ƶ�������b����a��
��2CO2+12H++12e��=CH3OCH3+3H2O
��3����̫���ܺ͵���ת��Ϊ��ѧ��
��2CO32��-4e��=2CO2![]() +O2
+O2![]()
3CO2+4e��=C+2CO32-
��������
�����������1��![]() 2��2��803+6��436-2��326-6��414-6��464=-92 KJ
2��2��803+6��436-2��326-6��414-6��464=-92 KJ![]() mol��1����2������̫���ܵ������Դ���ʴ�װ���ǵ��أ�����b�缫��ˮת��Ϊ����������������Ӧ��b�缫�������������ͷ�11.2L��������״���£���ת�Ƶĵ�������0.5��4mol=2mol������2mol H+�������ƶ�������������Ҳ��ƶ�������b����a���� ������a�ϵĵ缫��ӦʽΪ2CO2+12H++12e��=CH3OCH3+3H2O����3������ͼ��֪������������������ת����ʽ��̫���ܺ͵���ת��Ϊ��ѧ�ܣ���̼��Ʒֽ�ΪCaO��CO2�������Ϊ����̼���ƣ������ĵ缫��ӦʽΪ2CO32��-4e��=2CO2
mol��1����2������̫���ܵ������Դ���ʴ�װ���ǵ��أ�����b�缫��ˮת��Ϊ����������������Ӧ��b�缫�������������ͷ�11.2L��������״���£���ת�Ƶĵ�������0.5��4mol=2mol������2mol H+�������ƶ�������������Ҳ��ƶ�������b����a���� ������a�ϵĵ缫��ӦʽΪ2CO2+12H++12e��=CH3OCH3+3H2O����3������ͼ��֪������������������ת����ʽ��̫���ܺ͵���ת��Ϊ��ѧ�ܣ���̼��Ʒֽ�ΪCaO��CO2�������Ϊ����̼���ƣ������ĵ缫��ӦʽΪ2CO32��-4e��=2CO2![]() +O2
+O2![]() �������ĵ缫��ӦʽΪ3CO2+4e��=C+2CO32-��
�������ĵ缫��ӦʽΪ3CO2+4e��=C+2CO32-��

����Ŀ���±��г�������������Ԫ�������ڱ��е�λ�ã�
��A | ||||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
�밴Ҫ��ش��������⡣
��1��Ԫ������������______��Ԫ���������ڱ�������λ��___________��Ԫ�������⻯��е���������⻯��е㣬ԭ����_________________________________________��
��2���ܢޢ����⻯�ﰴ�ȶ���������ǿ��˳����________________________(д�⻯��Ļ�ѧʽ)��
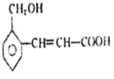
��3��Ԫ���ڢ��γ�ԭ�Ӹ�����Ϊ1:2�Ļ�����ĵ���ʽ�� ��
��4��Ԫ�����γɵ�һ���⻯���Ϊ������ͨʽ��___________������ �������ʽ��������ͬ���칹�壬�ṹ��ʽ�ֱ���____________________________________��
��5���õ���ʽ��ʾ�������γɻ�����Ĺ���_________________________��