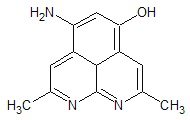
��Ŀ����
����Ŀ���״�(CH3OH)��һ���ж����ʣ����״������IJ����ǹ���ԭ��ʾ��ͼ���¡�����˵����ȷ���ǣ� ��
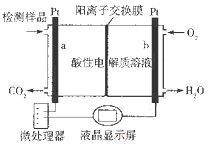
A. ��װ��Ϊ����ת��Ϊ��ѧ�ܵ�װ��
B. a�缫�����ĵ缫��ӦΪCH3OH + H2O - 6e-�T CO2�� + 6H+
C. ����·����1 mol e-ת��ʱ��������n(H��)����1 mol
D. �����Ե������Һ��Ϊ���Ե������Һ�ò����Dz����ܲ�������
���𰸡�B
�����������������A����װ��Ϊԭ��أ��ǽ���ѧ��ת��Ϊ���ܵ�װ�ã�A����B��a�缫��ԭ��صĸ����������ĵ缫��ӦΪ��CH3OH+H2O-6e-�TCO2��+6H+��B��ȷ��C�������ĵ缫��Ӧ�ǣ�O2+4H++4e-�TH2O������·����1 mol e��ת��ʱ��������n(H��)����1 mol��C����D�������Ե������Һ��Ϊ���Ե������Һ�ò�����һ����ԭ��أ�ͬ�����Բ���������D����ѡB

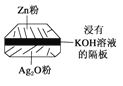
����Ŀ������ͭ���壬�׳����������������д��£�����ⶾ��ȡ5.0 g������Ʒ�������¶�ʹ��ֽ⣬�ֽ���̵��������±����ش��������⣺
�¶ȷ�Χ/�� | ��������/g |
258~680 | 3.20 |
680~1000 | 1.60 |
1000���� | 1.44 |
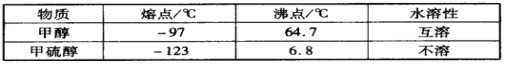
��1���ⶨ�������ھ��������������____________������SO42-��Sԭ�ӵĹ���ӻ���ʽ��____________��H2O�Ŀռ乹����__________________��
��2��������ͭ������ȵ�258~680�����ɵ�����A��A��__________����ѧʽ����A����ˮ�����Һ�����백ˮ���۲쵽��������_____________________________________________�����յõ���Һ�еĺ�ͭԪ�ص�������_________________����ѧʽ���������Ӻ��еĻ�ѧ��������___________________��
��3��������ͭ������ȵ�1000���������ɵ�����C����C�е�ͭ�����ӵĻ�̬�����Ų�ʽ��____________��
��4����ͼ������ͭ����ֽ�õ�һ���¶ȵIJ���ľ���������ͺ��������ͬ��ԭ������

�����¶���_______________��
��ͭԭ�ӵ���λ����_______________��
����֪�þ�����ܶ�Ϊdg��cm-3����������_________________pm��