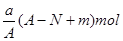��Ŀ����
����һ����֪��������þ�Ͻ����ⶨ����þ��������������λͬѧ������������ֲ�ͬ��ʵ�鷽��
ʵ�����1����þ�Ͻ� �ⶨ���ɵ������ڱ�״���µ����
�ⶨ���ɵ������ڱ�״���µ����
ʵ�����2����þ�Ͻ� �ⶨ���ɵ������ڱ�״���µ����
�ⶨ���ɵ������ڱ�״���µ����
ʵ�����3����þ�Ͻ� ��Һ
��Һ ���ˣ��ⶨ�õ�����������
���ˣ��ⶨ�õ�����������
���ܲⶨ��þ�������������ǣ� ��
A������ B�������� C�� �ٲ��ܣ��������� D�� �ڢ۲��ܣ� ����
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��300mL���ܱ������У��������۲�����һ������CO���壬һ�������·�����Ӧ��
Ni(s) + 4CO(g) Ni(CO)4(g)����֪�÷�Ӧƽ�ⳣ�����¶ȵĹ�ϵ���±���
Ni(CO)4(g)����֪�÷�Ӧƽ�ⳣ�����¶ȵĹ�ϵ���±���
����˵����ȷ���ǣ� ��
�¶�/�� | 25 | 80 | 230 |
ƽ�ⳣ�� | 5��104 | 2 | 1.9��10��5 |
A����������Ni(CO)4(g)�ķ�ӦΪ���ȷ�Ӧ
B��25��ʱ��ӦNi(CO)4(g) Ni(s) + 4CO(g)��ƽ�ⳣ��Ϊ0.5
Ni(s) + 4CO(g)��ƽ�ⳣ��Ϊ0.5
C����ij�����´ﵽƽ�⣬���Ni(CO)4��COŨ�Ⱦ�Ϊ0.5mol��L��1�����ʱ�¶ȸ���80��
D��80��ﵽƽ��ʱ������������䣬����ϵ�г���һ������CO���ٴδﵽƽ���CO�����������С



 B��OH- C��Fe3+ D��HCO3-
B��OH- C��Fe3+ D��HCO3- ��Ba(OH)2 C��Na2O2 D��Ar
��Ba(OH)2 C��Na2O2 D��Ar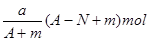 B.
B. 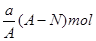
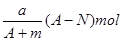 D.
D.