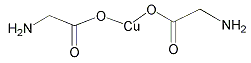��Ŀ����
�-������ͭ��������Ļ���������Cu4O��PO4��2����ͨ�����з�Ӧ�Ʊ���
2Na3PO4+4CuSO4+2NH3?H2O�TCu4O��PO4��2��+3Na2SO4+��NH4��2SO4+H2O
��1��д����̬Cu2+�ĺ�������Ų�ʽ��
��2��PO43-�Ŀռ乹����
��3���£�N2H4�����ӿ���ΪNH3�����е�һ����ԭ�ӱ�-NH2��������ȡ���γɵ���һ�ֵ����⻯�N2H4�����е�ԭ�ӹ�����ӻ�������
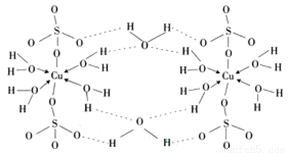
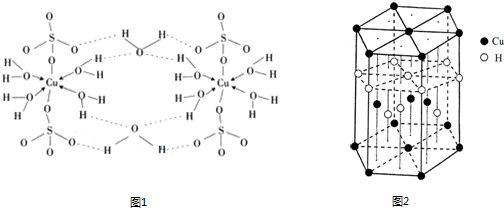
��4������CuSO4?5H2O�Ľṹʾ��ͼͼ1���京�е�������������
a�����Ӽ� b�����Լ� c�������� d����λ�� e����� f���Ǽ��Լ�
��5��������ͭ��Һ�м������KCN�����������[Cu��CN��4]2-����1mol CN-�к��еĦм�����ĿΪ
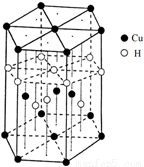
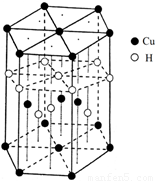
��6��CuԪ����HԪ�ؿ��γ�һ�ֺ�ɫ������侧��ṹ��Ԫ��ͼ2��ʾ����û�����Ļ�ѧʽΪ
2Na3PO4+4CuSO4+2NH3?H2O�TCu4O��PO4��2��+3Na2SO4+��NH4��2SO4+H2O

��1��д����̬Cu2+�ĺ�������Ų�ʽ��
[Ar]3d9
[Ar]3d9
����2��PO43-�Ŀռ乹����
��������
��������
����3���£�N2H4�����ӿ���ΪNH3�����е�һ����ԭ�ӱ�-NH2��������ȡ���γɵ���һ�ֵ����⻯�N2H4�����е�ԭ�ӹ�����ӻ�������
sp3
sp3
����4������CuSO4?5H2O�Ľṹʾ��ͼͼ1���京�е�������������
abde
abde
��������ţ�a�����Ӽ� b�����Լ� c�������� d����λ�� e����� f���Ǽ��Լ�
��5��������ͭ��Һ�м������KCN�����������[Cu��CN��4]2-����1mol CN-�к��еĦм�����ĿΪ
2NA��2mol
2NA��2mol
����6��CuԪ����HԪ�ؿ��γ�һ�ֺ�ɫ������侧��ṹ��Ԫ��ͼ2��ʾ����û�����Ļ�ѧʽΪ
CuH
CuH
����������1��ͭ��29��Ԫ�أ�����ͭ���Ӻ�����27�����ӣ����ݹ���ԭ��д�����������Ų�ʽ��
��2�����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
��3�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
��4������֮��������Ӽ����ǽ���Ԫ��֮���γɹ��ۼ������йµ��ӶԵ�ԭ�Ӻͺ��пչ����ԭ��֮�������λ������ԭ�Ӻ���ԭ�Ӽ���������
��5��һ�� CN-�к���2���м���
��6�����þ�̯��ȷ���仯ѧʽ��
��2�����ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
��3�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
��4������֮��������Ӽ����ǽ���Ԫ��֮���γɹ��ۼ������йµ��ӶԵ�ԭ�Ӻͺ��пչ����ԭ��֮�������λ������ԭ�Ӻ���ԭ�Ӽ���������
��5��һ�� CN-�к���2���м���
��6�����þ�̯��ȷ���仯ѧʽ��
����⣺��1��ͭ��29��Ԫ�أ�����ͭ���Ӻ�����27�����ӣ����ݹ���ԭ��֪�����������Ų�ʽΪ��[Ar]3d9���ʴ�Ϊ��[Ar]3d9��
��2��PO43-��Pԭ�ӵļ۲���Ӷ�=4+
(5+3-4��2)=4���Ҳ����µ��Ӷԣ�������ռ乹���������壬�ʴ�Ϊ���������壻
��3��N2H4�����е�ԭ�Ӻ���3�����۵������Ҳ����µ��Ӷԣ�����Nԭ�ӹ�����ӻ�����sp3���ʴ�Ϊ��sp3��
��4��ͭ���Ӻ����������֮��������Ӽ�����ԭ�Ӻ���ԭ�Ӽ���ڼ��Թ��ۼ���ͭԭ�Ӻ���ԭ�Ӽ������λ������ԭ�Ӻ���ԭ�Ӽ�����������ѡabde��
��5��һ�� CN-�к���2���м�������1mol CN-�к��еĦм�����ĿΪ��2NA��2mol���ʴ�Ϊ��2NA��2mol��
��6���þ����У�ͭԭ�Ӹ���=4+2��
+12��
=7��Hԭ�Ӹ���=1+3+6��
=7�������仯ѧʽΪCuH���ʴ�Ϊ��CuH��
��2��PO43-��Pԭ�ӵļ۲���Ӷ�=4+
| 1 |
| 2 |
��3��N2H4�����е�ԭ�Ӻ���3�����۵������Ҳ����µ��Ӷԣ�����Nԭ�ӹ�����ӻ�����sp3���ʴ�Ϊ��sp3��
��4��ͭ���Ӻ����������֮��������Ӽ�����ԭ�Ӻ���ԭ�Ӽ���ڼ��Թ��ۼ���ͭԭ�Ӻ���ԭ�Ӽ������λ������ԭ�Ӻ���ԭ�Ӽ�����������ѡabde��
��5��һ�� CN-�к���2���м�������1mol CN-�к��еĦм�����ĿΪ��2NA��2mol���ʴ�Ϊ��2NA��2mol��
��6���þ����У�ͭԭ�Ӹ���=4+2��
| 1 |
| 2 |
| 1 |
| 6 |
| 1 |
| 3 |
���������⿼�������ʽṹ�����ʣ���Щ֪ʶ�㶼�ǿ����ȵ㣬�ѵ��ǻ�ѧʽ��ȷ����ע�������ÿ�������ϵ�ԭ�ӱ�6������ռ�ж�����8������ռ�У�Ϊ�״��㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ