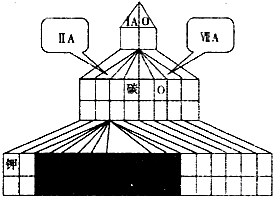
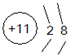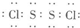��Ŀ����
���ж�����A��B��C����Ԫ�أ�ԭ��������������AԪ�صĵ������ܶ���С�����壬B���2�����ӿɴﵽ�ȶ��ṹ��C��Aͬ���壮
��1���ж�A��B��C��Ϊ����Ԫ�أ�дԪ�����ƣ���A
��2���õ���ʽ��ʾ����Ԫ��ԭ��֮����ܹ��ɵĻ�������γɹ��̣��������ۼ���ָ�����ۼ��ǦҼ����Ǧм����������ü��ĸ�����
��A��B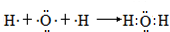 ������2���Ҽ���
������2���Ҽ���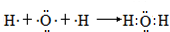 ������2���Ҽ�����
������2���Ҽ�����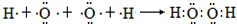 ������3���Ҽ���
������3���Ҽ���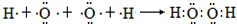 ������3���Ҽ���
������3���Ҽ���
��A��C �����Ӽ���
�����Ӽ��� �����Ӽ�����
�����Ӽ�����
��B��C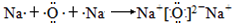
��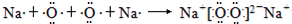 �����Ӽ������Ӽ������ۼ����к���1���Ҽ���
�����Ӽ������Ӽ������ۼ����к���1���Ҽ���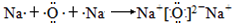
��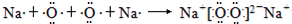 �����Ӽ������Ӽ������ۼ����к���1���Ҽ�����
�����Ӽ������Ӽ������ۼ����к���1���Ҽ�����
��1���ж�A��B��C��Ϊ����Ԫ�أ�дԪ�����ƣ���A
��
��
��B��
��
��C��
��
����2���õ���ʽ��ʾ����Ԫ��ԭ��֮����ܹ��ɵĻ�������γɹ��̣��������ۼ���ָ�����ۼ��ǦҼ����Ǧм����������ü��ĸ�����
��A��B
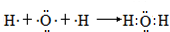 ������2���Ҽ���
������2���Ҽ���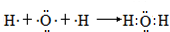 ������2���Ҽ���
������2���Ҽ���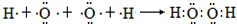 ������3���Ҽ���
������3���Ҽ���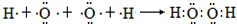 ������3���Ҽ���
������3���Ҽ�����A��C
 �����Ӽ���
�����Ӽ��� �����Ӽ���
�����Ӽ�����B��C
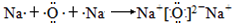
��
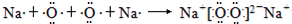 �����Ӽ������Ӽ������ۼ����к���1���Ҽ���
�����Ӽ������Ӽ������ۼ����к���1���Ҽ���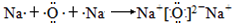
��
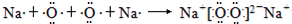 �����Ӽ������Ӽ������ۼ����к���1���Ҽ���
�����Ӽ������Ӽ������ۼ����к���1���Ҽ���������������A��B��C����Ԫ�أ�ԭ��������������AԪ�صĵ������ܶ���С�����壬��AΪ��Ԫ�أ�B���2�����ӿɴﵽ�ȶ��ṹ��B���ڵڢ�A�壬C��Aͬ���壬���ڢ�A��ԭ����������B����BΪ��Ԫ�ء�CΪNaԪ�أ��ݴ˽��
����⣺������A��B��C����Ԫ�أ�ԭ��������������AԪ�صĵ������ܶ���С�����壬��AΪ��Ԫ�أ�B���2�����ӿɴﵽ�ȶ��ṹ��B���ڵڢ�A�壬C��Aͬ���壬���ڢ�A��ԭ����������B����BΪ��Ԫ�ء�CΪNaԪ�أ�
��1��������������֪��AΪ��Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�
�ʴ�Ϊ���⣻�����ƣ�
��2����A��B�γ�ˮ��������⣬�γ�ˮ�Ĺ���Ϊ��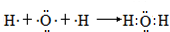 ������2���Ҽ�����
������2���Ҽ�����
�γɹ�������Ĺ���Ϊ��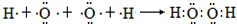 ������3���Ҽ�����
������3���Ҽ�����
�ʴ�Ϊ��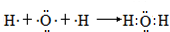 ������2���Ҽ�����
������2���Ҽ�����
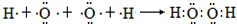 ������3���Ҽ�����
������3���Ҽ�����
��A��C�γ�NaH���γɹ���Ϊ�� �����Ӽ�����
�����Ӽ�����
�ʴ�Ϊ�� �����Ӽ���
�����Ӽ���
��B��C�γ���������������ƣ��γ������ƵĹ���Ϊ��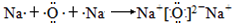 �����Ӽ�����
�����Ӽ�����
�γɹ������ƵĹ���Ϊ��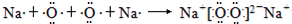 �����Ӽ������ۼ����к���1���Ҽ�����
�����Ӽ������ۼ����к���1���Ҽ�����
�ʴ�Ϊ��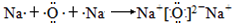 ��
��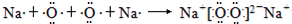 �����Ӽ������Ӽ������ۼ����к���1���Ҽ���
�����Ӽ������Ӽ������ۼ����к���1���Ҽ���
��1��������������֪��AΪ��Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�
�ʴ�Ϊ���⣻�����ƣ�
��2����A��B�γ�ˮ��������⣬�γ�ˮ�Ĺ���Ϊ��
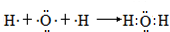 ������2���Ҽ�����
������2���Ҽ������γɹ�������Ĺ���Ϊ��
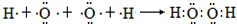 ������3���Ҽ�����
������3���Ҽ������ʴ�Ϊ��
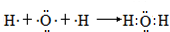 ������2���Ҽ�����
������2���Ҽ�����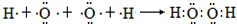 ������3���Ҽ�����
������3���Ҽ�������A��C�γ�NaH���γɹ���Ϊ��
 �����Ӽ�����
�����Ӽ������ʴ�Ϊ��
 �����Ӽ���
�����Ӽ�����B��C�γ���������������ƣ��γ������ƵĹ���Ϊ��
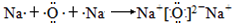 �����Ӽ�����
�����Ӽ������γɹ������ƵĹ���Ϊ��
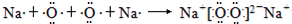 �����Ӽ������ۼ����к���1���Ҽ�����
�����Ӽ������ۼ����к���1���Ҽ������ʴ�Ϊ��
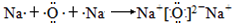 ��
��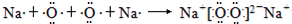 �����Ӽ������Ӽ������ۼ����к���1���Ҽ���
�����Ӽ������Ӽ������ۼ����к���1���Ҽ������������⿼�����ʽ��ʾ�������ѧ�����γɣ��Ѷ��еȣ�ע�����յ���ʽ����д��

��ϰ��ϵ�д�
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ