��Ŀ����
����Ŀ��ʵ�����Ʊ���ˮ�Ҵ���������ʵ�鲽�����¡�
��.��������ͼ��ʾ����100mLԲ����ƿ�м���10gС����״����ʯ�Һ������������ƣ�����ע��50mL��ҵ�Ҵ�������1h��
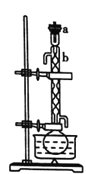
��.������ͼ��ʾ��������ϣ�����ƿ��ȴ������ƿ�м��뼸����ʯ����Ϊ����װ�ã��ռ�78��ʱ����֣���Ϊ��ˮ�Ҵ���(�á���ʾ�ƾ���)��
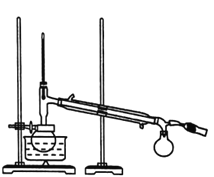
�Իش��������⣺
(1)װ��b��������________��
(2)��������ƿ�м�����ʯ�ҵķ���____________��
(3)����![]() ������Ϊ�˳�ȥ��ҵ�Ҵ���������ȩ�������ķ�ӦΪ
������Ϊ�˳�ȥ��ҵ�Ҵ���������ȩ�������ķ�ӦΪ![]() ���÷�Ӧ�ķ�Ӧ����Ϊ_______������1h��Ŀ����________��
���÷�Ӧ�ķ�Ӧ����Ϊ_______������1h��Ŀ����________��
(4)����ʱ�����¶ȼ�ʾ��Ϊ78������ʱ��β�ӹ��г���Һ�Σ���ȥ��ʼ������Һ�壬ԭ����_______��
(5)��ͬѧ���������Ϊ���ˣ���ش���˷�����ԭ����_____��
���𰸡�(����)������ ����ƿ��ţ���ֽ��(��ҩ��)���������뵽��ƿ�ײ���������ֱ����ƿ �ӳɷ�Ӧ ʹ![]() ��
��![]() ��ȩ��ȩ��ַ�Ӧ ��ʼ������Һ���к��еͷе������ ��������ȥ�ܽ����Ҵ��е��л����
��ȩ��ȩ��ַ�Ӧ ��ʼ������Һ���к��еͷе������ ��������ȥ�ܽ����Ҵ��е��л����
��������
(1)���������Ľṹ��֪��װ��b��������(����)�����ܣ��ʴ�Ϊ��(����)�����ܣ�
(2)����ҩƷ���ӹ����֪������ƿ�м�����ʯ�ҵķ����ǽ���ƿ��ţ���ֽ��(��ҩ��)���������뵽��ƿ�ײ���������ֱ����ƿ���ʴ�Ϊ������ƿ��ţ���ֽ��(��ҩ��)���������˵���ƿ�ײ���������ֱ����ƿ��
(3)��Ӧ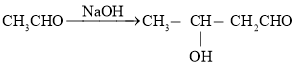 Ϊȩ���ļӳɷ�Ӧ���������Իӷ������ʻص���ƿ�м������뷴Ӧ��ʹ
Ϊȩ���ļӳɷ�Ӧ���������Իӷ������ʻص���ƿ�м������뷴Ӧ��ʹ![]() ��
��![]() ��ȩ��ȩ��ַ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��ʹ
��ȩ��ȩ��ַ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��ʹ![]() ��
��![]() ��ȩ��ȩ��ַ�Ӧ��
��ȩ��ȩ��ַ�Ӧ��
(4)���ڿ�ʼ������Һ���к��еͷе�����ʣ����������ʱ�����¶ȼ�ʾ��Ϊ78������ʱ��β�ӹ��г���Һ�Σ�Ҫ��ȥ��ʼ������Һ�壻�ʴ�Ϊ����ʼ������Һ���к��еͷе�����ʣ�
(5)��������ȥ�ܽ����Ҵ��е��л���ȣ��ʹ��˷����ף��ʴ�Ϊ����������ȥ�ܽ����Ҵ��е��л��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�