��Ŀ����
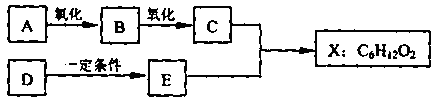
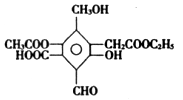
����ͼ��X����֧���ġ����й���ζ�ĺϳ����ϣ������ڵ�����ֹ������㾫����֪
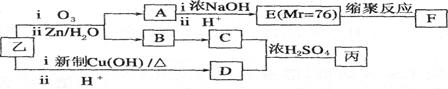
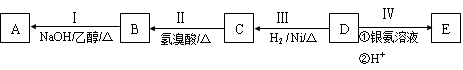
D�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ������ʯ�ͻ�����չˮƽ��E������
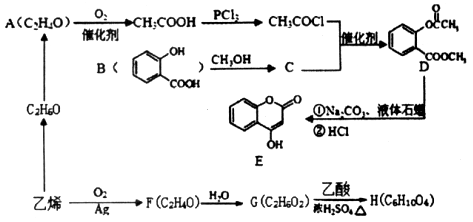
�г�����һ���л�������ʼ�ת����ϵ���£�
��ش��������⡣
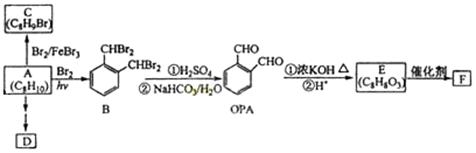
(1)A�������� ��
(2)B�������������� ��
(3)C+E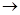 X�Ļ�ѧ��Ӧ������ ��Ӧ��
X�Ļ�ѧ��Ӧ������ ��Ӧ��
(4)д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ (����A)�� ��
(5)X������������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
(6)��DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ�� ��
D�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ������ʯ�ͻ�����չˮƽ��E������
�г�����һ���л�������ʼ�ת����ϵ���£�

��ش��������⡣
(1)A�������� ��
(2)B�������������� ��
(3)C+E
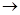 X�Ļ�ѧ��Ӧ������ ��Ӧ��
X�Ļ�ѧ��Ӧ������ ��Ӧ��(4)д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ (����A)�� ��
(5)X������������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
(6)��DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ�� ��
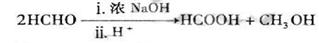
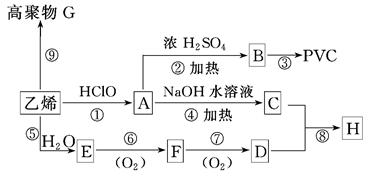
��1��1������ ��2��ȩ�� ��3��������Ӧ����ȡ����Ӧ��
��4��(CH3)3COH��CH3CH2CH(OH)CH3��(CH3)2CHCH2OH
��5��CH3CH2CH2COOCH2CH3��NaOH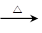 CH3CH2CH2COONa��CH3CH2OH
CH3CH2CH2COONa��CH3CH2OH
��6��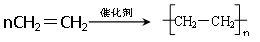
��4��(CH3)3COH��CH3CH2CH(OH)CH3��(CH3)2CHCH2OH
��5��CH3CH2CH2COOCH2CH3��NaOH
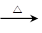 CH3CH2CH2COONa��CH3CH2OH
CH3CH2CH2COONa��CH3CH2OH��6��

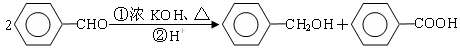
���������D�IJ���������������һ������ʯ�ͻ�����չˮƽ����D����ϩ��A��������B��B��������C��C��E��Ӧ���ɾ��й���ζ�ĺϳ����ϣ���˵��XӦ�������࣬����C�����ᣬE�Ǵ�����˵��E����ϩ��ˮ��ӳɷ�Ӧ���ɵ��Ҵ��������ԭ���غ��֪��CӦ���Ƕ��ᡣ����X����֧���ģ�����B�Ƕ�ȩ����A������������1��������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿���������ǿ����ע�ض�ѧ������֪ʶ������ѵ����ͬʱ�����ض�ѧ����������������ⷽ����ָ����ѵ��������Ĺؼ��Ǽ�ס���������ŵĽṹ�������Լ�������֮����ת����Ȼ��������������ü��ɡ�

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ
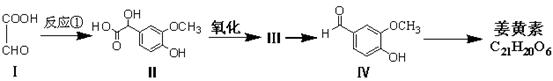
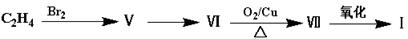
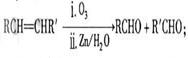
 ��
��
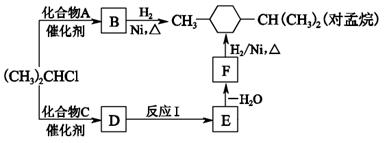
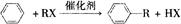 ��RΪ�����XΪ±��ԭ�ӣ���
��RΪ�����XΪ±��ԭ�ӣ���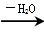 RCH=CH2
RCH=CH2