��Ŀ����
������һ���ḻ���ʱ��⣬ͨ����ˮ���ۺ����ÿɻ���������ʹ�����ʹ�á�
��1����ˮ���εĿ������ã�
�ٺ�ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���뺣�ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ� �ء�
��Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ�����ã� ��дһ�㼴�ɣ���
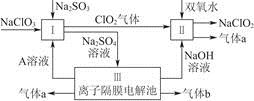
��2�����������ǽ��귢չ���һ�ֽϺõĺ�ˮ������������ԭ����ͼ��ʾ�����о���ѡ���Ե������ӽ���Ĥ�������ӽ���Ĥ������С���ش���������⣺
�ٺ�ˮ����ֱ��ͨ�뵽�������У������� ��
��A���ų����� �����ˮ����Ũˮ������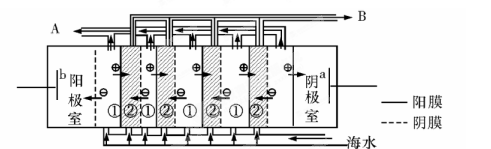
��3���ÿ�±���� �����ӣ�����ȡ�壬�������������£�
�����ӣ�����ȡ�壬�������������£�
�����������е���Һ��BrO3-�����������з�Ӧ�����ӷ���ʽΪ�� ��
��ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ���»�ú�Br2����Һ�� ��
������������ͨ��ˮ�������ȣ������¶���90�����ҽ��������ԭ���� ��
��1���ٽᾧ ����ֹH2��Cl2������Ӧ����������ը����ֹCl2�����ɵ�NaOH��Һ��Ӧ��ʹ�ռ��Ʒ�����ȣ�����������Ҳ���֣�
��2���ٺ�ˮ�к��϶�Mg2+��Ca2+�������ӣ����ʱ�����Mg��OH��2��Ca��OH��2�ȳ����Ӷ����������ӽ���Ĥ������������Ҳ���֣���3�֣��ڵ�ˮ
��3����3CO32-+3Br2=5Br-+BrO3-+3CO2��
�ڸ����壬���Br2��Ũ�ȣ�����������Ҳ���֣�
���¶ȹ������Խ�Br2������������¶ȹ����ֻὫ������ˮ�������
���������������1���ٺ�ˮ������Ҫ�������������ᾧԭ���������������Ϊ��ˮ�ء������غͽᾧ�ء����ȼ����������������ڻ�����������������������ӽ���Ĥ�����ã���ֹH2��Cl2������Ӧ����������ը����ֹCl2�����ɵ�NaOH��Һ��Ӧ��ʹ�ռ��Ʒ�����ȡ�
��2���ٺ�ˮ�к��϶�Mg2+��Ca2+�������ӣ����ʱ�����Mg��OH��2��Ca��OH��2�ȳ����Ӷ����������ӽ���Ĥ���ʺ�ˮ����ֱ��ͨ�뵽�������С���A���ų����ǵ�ˮ��
��3�����ÿ�±��ȡ�壬�������з����ķ�ӦΪ����̼���Ƶķ�Ӧ���������к�BrO3-����Ӧ����ʽΪ3CO32-+3Br2=5Br-+BrO3-+3CO2������ͨ�����Ȼ��ѻ�ú�Br2����Һ�������Ũ��̫�ͣ��������ռ����������������ա��ữ���»�ú�Br2����Һ��Ϊ�˸����壬���Br2��Ũ�ȣ����¶ȹ������Խ�Br2������������¶ȹ����ֻὫ������ˮ���������������������ͨ��ˮ�������ȣ������¶���90�����ҽ�������
���㣺���麣ˮ���Ρ���ˮ��������ˮ��������֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д����о��ϳɰ���������ʷ�ϣ���ͬ���о�����3�λ�ŵ������ѧ�����ϳɰ���������ũ����IJ�����ͬʱҲ����ȡ���ᡢըҩ�ȵ�ԭ�ϡ�����˵������ȷ����
| A��N2��H2�ڵ�ȼ����������¿ɺϳɰ��� |
| B������HNO3���ǵ���� |
| C����������Ũ����ᷢ����Ӧ������������ |
| D����NH3��HNO3�Ĺ����У���Ԫ�ر���ԭ |
���ж���ʵ�Ľ��Ͳ���ȷ����
| ѡ�� | ��ʵ | ���� |
| A | �����۳�����Ũ���� | Ũ�����ǿ������ʹ���ۻ� |
| B | �ñ���NH4Cl��Һ����������̨�����ɷ��� | NH4Cl�ֽ����ȣ��ҷֽ�����ܸ��������е����� |
| C | ��Ԫ�ص���ɫ��Ӧ�ʻ�ɫ | Na2O2��һ�ֵ���ɫ�Ĺ��� |
| D | SO2��ʹ��ˮ��ɫ | SO2���л�ԭ�� |
����ѧ��ѡ��2����ѧ�뼼����(15��)
I����ˮ����һ����������������������Cu2����Hg2����Pb2�����ؽ������ӣ��ɼ��������ʹ��������������ʲ�����Ϊ����������
| A����ˮ | B���������� | C����������Һ | D��������Һ |
�ش��������⣺
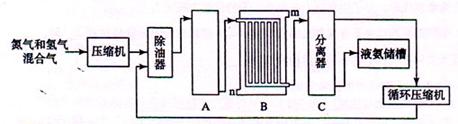
��1����ҵ�ϳɰ���ԭ���ǵ����������������Ǵӿ����з�������ģ�ͨ��ʹ�õ����ַ��뷽���� �� ����������Դ��ˮ��̼�⻯���д���ֱ����ú����Ȼ��Ϊԭ����ȡ�����Ļ�ѧ��Ӧ����ʽ ��
��
��2�� �豸A�к��е����������ú���Ƚ��������豸A������ �����з����Ļ�ѧ��Ӧ����ʽΪ ��
��3�� �豸B������ ������m��n������ͨˮ�ڣ���ˮ���� (�m����n��)�����˴��෴����ͨˮ��ԭ�� ��
��4�� �豸C������ ��
��5����ԭ�����Ʊ������л���CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�CO(g)��H2O(g)
 CO2 (g)�� H2 (g) �� ��֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)������ ��
CO2 (g)�� H2 (g) �� ��֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)������ �� (4�֣���1��д���������ʵĻ�ѧʽ����Ư�۵���Ч�ɷ֣� ���ڱ��ͣ� ��
��2��д����Na2SO3��ŨH2SO4��Ӧ�Ʊ�SO2�Ļ�ѧ����ʽ�� ��
����ѧ��ѡ��2����ѧ�뼼����(15��)
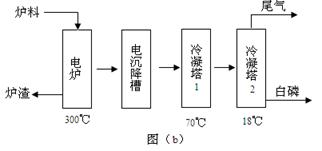
��ʯ��Ҫ������ơ�Ca3(PO4)2��H2O������ʯ��Ca3(OH)(PO4)3������ʽ���ڡ�ͼ(a)ΪĿǰ��������ʯ���õĴ������������ʪ��������ָ��ʯ�ù�������ֽ��Ʊ����ᡣͼ(b)���ȷ�������������������ʯ�Ƶ��������̡�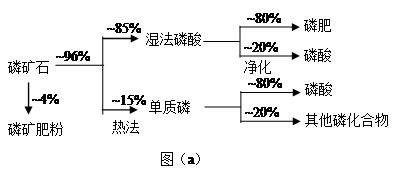
�������ʵ�����������£�
| | �۵�/�� | �е�/�� | ��ע |
| ���� | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ������ˮ�����л�ԭ�� |
| SiF4 | -90 | -86 | ��ˮ�� |
��1����������ʯ����Ҫ����;�����������ϣ�Լռ��ʯʹ������ �G��
��2������ʯΪԭ�ϣ�ʪ�����������Ca3F(PO4)3��Ӧ�Ļ�ѧ����ʽΪ�� ������1���ۺϺ�������������Լ30%����ʯ�������Ƶ�85�G����Ʒ���� �֡�
��3����ͼ(b)��ʾ���ȷ���������ĵ�һ���ǽ��������衢������̿����ʯ��ϣ����·�Ӧ���ɰ��ס�¯������Ҫ�ɷ��ǣ� (�ѧʽ)������1����Ҫ�������ǣ� ������2����Ҫ�������ǣ�
��4��β������Ҫ���� ������������PH3��H2S��HF�ȣ���β����ͨ�봿����Һ���ɳ�ȥ
��ͨ�����������Һ���ɳ�ȥ (���ѧʽ)
��5�������ʪ�����ᣬ�ȷ����Ṥ�ո��ӣ��ܺĸߣ����ŵ��ǣ� ��
��������һ�ָ����մɲ��ϣ���Ӳ�ȴ��۵�ߣ���ѧ�����ȶ�����ҵ���ձ���øߴ����봿����1 300 �淴Ӧ��á�
(1)�������ʣ��Ʋ�����մɵ���;��________��(�����)
| A�������ֻ� | B������ɫ���� |
| C����������ģ�� | D��������ͻ� |
(3)�������մɿ���ʴ����ǿ����������⣬�������������ᷴӦ�����Ʋ���մɱ�����ḯʴ�Ļ�ѧ����ʽ___________________________________��
(4)�������Ȼ����뵪�����������ձ����£���ǿ�ȷ�����Ӧ���ɵýϸߴ��ȵĵ����裬��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��