��Ŀ����
1��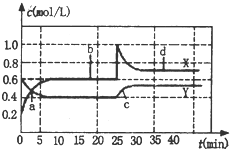 ��֪NO2��N2O4�����ת����2NO2 ��g��?N2O4 ��g����H��0���ֽ�һ����NO2��N2O4�Ļ�����壬ͨ�����Ϊ1L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵��������ǣ�������
��֪NO2��N2O4�����ת����2NO2 ��g��?N2O4 ��g����H��0���ֽ�һ����NO2��N2O4�Ļ�����壬ͨ�����Ϊ1L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵��������ǣ�������| A�� | ͼ�й�����������X��Y����������X��ʾ NO2Ũ����ʱ��ı仯 | |
| B�� | a��b��c��d�ĸ����У���ʾ��ѧ��Ӧ����ƽ��״̬�ĵ���b��d | |
| C�� | ��Ҫ�ﵽ��d��ͬ��״̬����25minʱ���ܲ�ȡ�Ĵ�ʩ���ʵ���С������� | |
| D�� | ��Ӧ������25minʱ�����߷����仯��ԭ���Ǽ���0.4 mol N2O4 |
���� A����ͼ�����߱仯��֪��XΪNO2�ı仯���ߣ�YΪN2O4�ı仯���ߣ�
B���ﵽƽ���ʱ���Ϊ10min��25min�Լ�30min�Ժ�ʾ��ѧ��Ӧ����ƽ��״̬�ĵ���b��d��
C��d״̬Ϊƽ��״̬��NO2��N2O4��Ũ����ԭ����ƽ��״̬��ȽϾ������ʵ���С���������NO2��N2O4��Ũ�ȶ����ɴﵽ��d��ͬ��״̬��
D��25minʱ��NO2��Ũ������0.4mol������ӦΪ����0.4mol��NO2��
��� �⣺A����ͼ�����߱仯��֪����10minʱ��XŨ�ȱ仯��Ϊ0.4mol/L��YŨ�ȱ仯��Ϊ0.2mol/L����XΪNO2�ı仯���ߣ�YΪN2O4�ı仯���ߣ���A��ȷ��
B���ﵽƽ���ʱ���Ϊ10min��25min�Լ�30min�Ժ�ʾ��ѧ��Ӧ����ƽ��״̬�ĵ���b��d����B��ȷ��
C��d״̬Ϊƽ��״̬��NO2��N2O4��Ũ����ԭ����ƽ��״̬��ȽϾ������ʵ���С���������NO2��N2O4��Ũ�ȶ����ɴﵽ��d��ͬ��״̬����C��ȷ��
D��25minʱ��NO2��Ũ������0.4mol������ӦΪ����0.4mol��NO2����D����
��ѡD��
���� ���⿼�黯ѧƽ��ͼ�����⣬��Ŀ�Ѷ��еȣ�����ע��������ߵı仯���ƣ���ȷ�ж����������ƽ���ƶ���Ӱ�죮

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ
11�������������ʵ����ͬһ��ѧԭ������˵�����ǣ�������
| A�� | �Ȼ�狀͵ⶼ�����ü��ȷ������ᴿ | |
| B�� | Na��Mg��ˮ��Ӧ����Һ�е����̪��Һ���ɫ | |
| C�� | ����������Һ��ˮ�����ڿ����о��ú������� | |
| D�� | ��ˮ�Ͷ��������������ʹƷ����Һ��ɫ |
16����ȷ�Ϸ����˻�ѧƽ���ƶ����ǣ�������
| A�� | �ı�ijƽ����ϵ���¶� | |
| B�� | ����̬���ʲμӵĿ��淴Ӧ�ﵽƽ��ı�ѹǿ | |
| C�� | ʹƽ�������и���ֵ�Ũ�ȷ����仯 | |
| D�� | ���淴Ӧ�ﵽƽ���ʹ�ô��� |
6�������£���10ml pH=10 ��NaOH ��Һ����μ��� pH=4��һԪ��HA��Һ��pH�պõ���7�����跴Ӧǰ����Һ������伴��Һ����ܼ���ӣ�����Է�Ӧ����Һ��������ȷ���ǣ�������
| A�� | c��A-����c��Na+�� | B�� | c��H+��=c��OH-����c��Na+����c��A-�� | ||
| C�� | V���죩��20mL | D�� | V���죩��20mL |
13��2SO2��g��+O2��g��$\frac{\underline{\;V_{2}O_{5}\;}}{\;}$2SO3��g����H��0���Ʊ��������Ҫ��Ӧ�����й��ڸ÷�Ӧ����������ȷ���ǣ�������
| A�� | �����¶Ƚ����̷�Ӧ�ﵽƽ���ʱ�� | |
| B�� | ����Ӧ��ϵ��ѹǿ��Ӧ����һ������ | |
| C�� | ����V2O5ͬʱ�ı����淴Ӧ���� | |
| D�� | ����O2��Ũ�Ƚ����SO2��ת���� |
10����ͼ���йص���������ȷ���ǣ�������
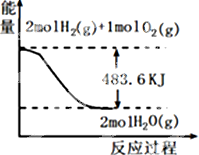

| A�� | ��ʾ1mol H2��g����ȫȼ������ˮ��������241.8 kJ���� | |
| B�� | ��ʾ���Ȼ�ѧ����ʽΪ��H2��g��+$\frac{1}{2}$ O2��g��=H2O��g����H=-241.8 kJ•mol-1 | |
| C�� | H2O��g������������H2��g����O2��g��������֮�� | |
| D�� | ��ʾ2 mol H2��g�������е�����һ����2 mol��̬ˮ�����е�������483.6 kJ |
11����һ���̶�������ܱ������м���2molA���������淴Ӧ��2A��g��?2B��g��+C��g�����÷�Ӧ�ﵽƽ��ı�־�ǣ�������
| A�� | ������ѹǿ�ǿ�ʼʱ��1.5�� | |
| B�� | ������A��B��C���ʵ���Ũ��֮��Ϊ2��2��1 | |
| C�� | ��λʱ������0.2 mol A ͬʱ����0.1 mol C | |
| D�� | �����ڸ����ʵ�Ũ�Ȳ���ʱ��仯 |
 ��ϩ�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��������Ϊ��ϩ�ܹ�ת��Ϊ������Ҫ���л�����ԭ�ϣ����磺
��ϩ�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��������Ϊ��ϩ�ܹ�ת��Ϊ������Ҫ���л�����ԭ�ϣ����磺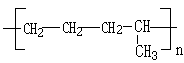 ��
�� ��
�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O��