��Ŀ����
16�� �״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϣ���ҵ�Ͽ�����CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
�״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϣ���ҵ�Ͽ�����CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��| ��ѧ��Ӧ | ƽ�ⳣ�� | �¶ȡ� | |
| 500 | 800 | ||
| ��2H2��g��+CO��g��?CH3OH��g�� | K1 | 2.5 | 0.15 |
| ��H2��g��+CO2��g��?H2O ��g��+CO��g�� | K2 | 1.0 | 2.50 |
| ��3H2��g��+CO2��g��?CH3OH��g��+H2O ��g�� | K3 | ||
���ʱ V���� V�������������=����������
��2����3L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c��CO��-��Ӧʱ��t�仯���ߢ���ͼ��ʾ������t0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ�
�����ߢ��Ϊ���ߢ�ʱ���ı�������Ǽ��������
�����ߢ��Ϊ���ߢ�ʱ���ı�������ǽ��������������ѹ����2L��
��3��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᣮͨ��״���£���a mol/L�Ĵ�����b mol/LBa��OH��2��Һ�������ϣ���Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-�����ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ$\frac{2b}{a-2b}$��10-7��
���� ��1��������Ӧ������֪ƽ�ⳣ��K3=K1��K2�����㲻ͬ�¶��·�Ӧ�۵�ƽ�ⳣ��������¶ȱ仯�����жϷ�Ӧ�ʱ��H��0�����ݷ�Ӧ��+�ڵõ���Ӧ�ۣ�����ƽ�ⳣ��K3=K1��K2������ijʱ��Ũ���̼����ƽ�ⳣ���Ƚ��жϷ�Ӧ���еķ���
��2��ͼ��������ߢ�仯Ϊ���ߢ������̷�Ӧ�ﵽƽ���ʱ�䣬���ﵽ��ͬƽ��״̬������ǿɱ���Ǻ�ѹ������˵���ı���Ǽ����˴����������ߢ��Ϊ���ߢ�ʱһ����̼���ʵ�������Ӧ�������������ķ�Ӧ���ɱ����������������Ũ�ȳɷ��ȣ��������ʵ������䣻
��3����Һ������������Ũ�ȼ���һ�룬�������ƽ�ⳣ����Ũ���أ�ͨ��״���£���a mol/L�Ĵ�����b mol/L Ba��OH��2��Һ�������ϣ���Һ������Ϊ���ᱵ��������������Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-��=bmol/L����Һ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ���ϵ���ƽ�ⳣ��������㣮
��� �⣺��1����Ӧ��3H2��g��+CO2��g���TCH3OH��g��+H2O��g�������������С�ķ�Ӧ��S��0�����ݷ�Ӧ��+�ڵõ���Ӧ�ۣ�����ƽ�ⳣ��K3=K1��K2������¶ȱ仯���������¶����ߣ�ƽ�ⳣ����С��ƽ��������У������жϷ�Ӧ�Ƿ��ȷ�Ӧ���ʱ��H��0����500�桢2L���ܱ������У����з�Ӧ�ۣ���÷�Ӧ����ijʱ�̣�H2��g����CO2��g����CH3OH��g����H2O ��g����Ũ�ȣ�mol/L���ֱ�Ϊ0.8��0.1��0.3��0.15��Q=$\frac{0.15��0.3}{0��{8}^{3}��0.1}$=0.87��K=2.5����Ӧ������У�V����V�棬
�ʴ�Ϊ��K1•K2������
��2��ͼ��������ߢ�仯Ϊ���ߢ������̷�Ӧ�ﵽƽ���ʱ�䣬���ﵽ��ͬƽ��״̬������ǿɱ�ģ��Ǻ�ѹ������˵���ı���Ǽ����˴�����
�����ߢ��Ϊ���ߢ�ʱһ����̼���ʵ�������Ӧ�������������ķ�Ӧ���ɱ����������������Ũ�ȳɷ��ȣ��������ʵ������䣬���ߢ����Ϊ3L��һ����̼Ũ��Ϊ3mol/L���ı����������ߢ��Ϊ���ߢ�ʱ��һ����̼Ũ��Ϊ4.5mol/L�������ѹ�����Ϊ��3��V=4.5��3��V=2L�����Խ��������������ѹ����2L���ϣ�
�ʴ�Ϊ��������������������������ѹ����2L��
��5��ͨ��״���£���a mol/L�Ĵ�����b mol/L Ba��OH��2��Һ�������ϣ���Һ������Ϊ���ᱵ��������������Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-��=bmol/L����Һ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ��������ƽ�ⳣ�����ݵ��뷽��ʽд��K=$\frac{c��C{H}_{3}CO{O}^{-}��c��{H}^{+}��}{c��C{H}_{3}COOH��}$=$\frac{b��1{0}^{-7}}{\frac{a}{2}-b}$=$\frac{2b}{a-2b}$��10-7L/mol��
�ʴ�Ϊ��$\frac{2b}{a-2b}$��10-7L/mol��
���� ���⿼���˻�ѧƽ��Ӱ�����ط����жϣ�ƽ�ⳣ�������Ӱ��������Ӧ�ã����ܵ������Һ���ܶȻ��ļ��㣬��Ŀ�ۺ��Խϴ�

 ������ѧ��ѧԭ��������������⣺
������ѧ��ѧԭ��������������⣺��1���軯�����������ˮ���о綾������HCN���ӷ������ᣬ��֪��Ka��HCN��=6.17x10-10��������CN-��ˮʱ����NaOH��Һ������pH=9ʱ�����£���c��CN-����c��HCN�������������������=������
��2����֪��
��C��s��+O2��g���TCO2��g������H=a kJ•mol-1��
��CO2��g��+C��s���T2CO��g������H=b kJ•mol-1��
��Si��s��+O2��g���TSiO2��s������H=c kJ•mol-1��
��ҵ�������ֹ���Ȼ�ѧ����ʽΪ2C��s��+SiO2��s���TSi��s��+2CO��g����H=��a+b-c��kJ•mol-1��
��3����֪��CO��g��+H2O��g��?H2��g��+CO2��g�����±�Ϊ�÷�Ӧ�ڲ�ͬ�¶�ʱ��ƽ�ⳣ����
| �¶�/ | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
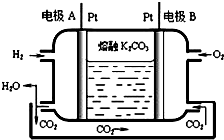
��7��һ����������ȼ�ϵ�ع���ԭ����ͼ��ʾ��д���缫A�ĵ缫��ӦʽH2-2e-+CO32-�TCO2+H2O��

| A�� | Ǧ���ص�A��Ϊ�������缫����ΪPb | |
| B�� | Ǧ���ع���������ÿͨ����·��2mol����������1molH2SO4 | |
| C�� | �õ��ص�������ӦΪ 2NO3-+6H2O+10e-=N2��+12OH- | |
| D�� | ����������ת��5moL���ӣ���Ĥ������Һ�������仯���m��-��m����Ϊ14.4g |
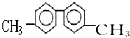
| A�� | ���������������6��̼ԭ�Ӵ���ͬһֱ���� | |
| B�� | ����������������10��̼ԭ�Ӵ���ͬһƽ���� | |
| C�� | ������һ�ȴ������������ | |
| D�� | �����DZ���ͬϵ���ʹ���Ը��������Һ��ɫ |
| A�� | �٢ڢ� | B�� | ֻ�Тڢۢ� | C�� | �ڢۢܢ� | D�� | ȫ������ |
 1��2һ������ϩ����ͼ���ֽṹ��
1��2һ������ϩ����ͼ���ֽṹ��