��Ŀ����
����Ŀ����֪��![]() ��
��![]()
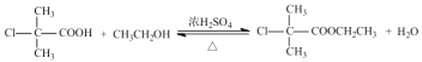
I.����ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·��������
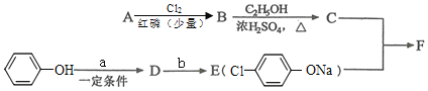
��1��AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ����2.24LCO2(��״��)��A�ķ���ʽΪ____________��
��2��д������A����ʽ�����м������Ľṹ��ʽ��________________��
��3��B���ȴ����ᣬ��˴Ź��������������壬д��B��C�ķ�Ӧ����ʽ��____________��
��4��C+E��F�ķ�Ӧ����Ϊ____________��
��5��д��A��F�Ľṹ��ʽ��____________��
��6��D�ı������������⣬�����������ŵ�����Ϊ_____________��д��b���������Լ�Ϊ____________��
II.�л���H�ķ���ʽΪC2H2O3���ɷ���������Ӧ���Ҿ������ԡ�
��7��H�ж��ֺϳɷ������ڷ�����д��������ϳ�H��·������ͼ(����ԭ����ѡ)���ϳ�·������ͼʾ��������![]() ________________________________________________��
________________________________________________��
���𰸡� C4H8O2 ![]()
![]()
 ȡ����Ӧ
ȡ����Ӧ ![]()
 �ǻ�����ԭ�� NaOH��Һ
�ǻ�����ԭ�� NaOH��Һ ![]()
��������������AΪһԪ������n��CO2��=![]() =0.1mol����n��A��=0.1mol��M��A��=
=0.1mol����n��A��=0.1mol��M��A��=![]() =88g/mol���òл����෨��
=88g/mol���òл����෨��![]() =3����7��A�ķ���ʽΪC4H8O2��A��B���������֪��ȡ����Ӧ��B���ȴ�������B�ĺ˴Ź��������������壬B�Ľṹ��ʽΪ
=3����7��A�ķ���ʽΪC4H8O2��A��B���������֪��ȡ����Ӧ��B���ȴ�������B�ĺ˴Ź��������������壬B�Ľṹ��ʽΪ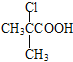 ��A�Ľṹ��ʽΪ
��A�Ľṹ��ʽΪ![]() ��B��CH3CH2OH����������Ӧ����C��C�Ľṹ��ʽΪ
��B��CH3CH2OH����������Ӧ����C��C�Ľṹ��ʽΪ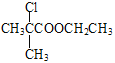 ������
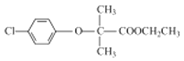
������![]() ��D��E��E�Ľṹ��ʽ��D�ı������������⣬D�Ľṹ��ʽΪ
��D��E��E�Ľṹ��ʽ��D�ı������������⣬D�Ľṹ��ʽΪ![]() ��E��C���������֪�ķ�Ӧ��F�Ľṹ��ʽΪ
��E��C���������֪�ķ�Ӧ��F�Ľṹ��ʽΪ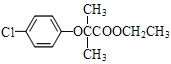 ��
��
�����AΪһԪ������n��CO2��=![]() =0.1mol����n��A��=0.1mol��M��A��=
=0.1mol����n��A��=0.1mol��M��A��=![]() =88g/mol���òл����෨��
=88g/mol���òл����෨��![]() =3����7��A�ķ���ʽΪC4H8O2��A��B���������֪��ȡ����Ӧ��B���ȴ�������B�ĺ˴Ź��������������壬B�Ľṹ��ʽΪ
=3����7��A�ķ���ʽΪC4H8O2��A��B���������֪��ȡ����Ӧ��B���ȴ�������B�ĺ˴Ź��������������壬B�Ľṹ��ʽΪ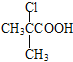 ��A�Ľṹ��ʽΪ
��A�Ľṹ��ʽΪ![]() ��B��CH3CH2OH����������Ӧ����C��C�Ľṹ��ʽΪ
��B��CH3CH2OH����������Ӧ����C��C�Ľṹ��ʽΪ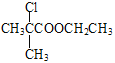 ������
������![]() ��D��E��E�Ľṹ��ʽ��D�ı������������⣬D�Ľṹ��ʽΪ
��D��E��E�Ľṹ��ʽ��D�ı������������⣬D�Ľṹ��ʽΪ![]() ��E��C���������֪�ķ�Ӧ��F�Ľṹ��ʽΪ
��E��C���������֪�ķ�Ӧ��F�Ľṹ��ʽΪ ��
��
��1����������������A�ķ���ʽΪC4H8O2��
��2������A����ʽ�����м������Ľṹ��ʽΪ��HCOOCH2CH2CH3��![]() ��
��
��3��B�Ľṹ��ʽΪ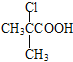 ��B��CH3CH2OH����������Ӧ����C��B��C�Ļ�ѧ����ʽΪ��
��B��CH3CH2OH����������Ӧ����C��B��C�Ļ�ѧ����ʽΪ��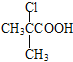 +CH3CH2OH
+CH3CH2OH![]()
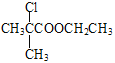 +H2O��
+H2O��
��4���Ա�C��E��F�Ľṹ��ʽ��C+E��FΪȡ����Ӧ��
��5��������������A�Ľṹ��ʽΪ![]() ��F�Ľṹ��ʽΪ
��F�Ľṹ��ʽΪ ��
��
��6��D�ı������������⣬D�Ľṹ��ʽΪ![]() ��D�����������ŵ�����Ϊ���ǻ�����ԭ�ӡ��Ա�D��E�Ľṹ��ʽ��b������ǻ���Ӧ���ɷ��ƣ�b���������Լ�ΪNaOH��Һ��Na2CO3��Һ��
��D�����������ŵ�����Ϊ���ǻ�����ԭ�ӡ��Ա�D��E�Ľṹ��ʽ��b������ǻ���Ӧ���ɷ��ƣ�b���������Լ�ΪNaOH��Һ��Na2CO3��Һ��
��7��H�ķ���ʽΪC2H2O3��H�ɷ���������Ӧ�Ҿ������ԣ�H�Ľṹ��ʽΪ![]() ���Ա�CH3COOH��
���Ա�CH3COOH��![]() �Ľṹ��̼�ɹǼ�û�б仯���Ȼ����䣬�ڼ������뺬�������ţ���������֪�Լ��л���֮���ת����ϵ��CH3COOH��Cl2�ں��ף�����������ʱ����ȡ����Ӧ����
�Ľṹ��̼�ɹǼ�û�б仯���Ȼ����䣬�ڼ������뺬�������ţ���������֪�Լ��л���֮���ת����ϵ��CH3COOH��Cl2�ں��ף�����������ʱ����ȡ����Ӧ����![]() ��
��![]() ��NaOHˮ��Һ�з���ˮ�ⷴӦ����
��NaOHˮ��Һ�з���ˮ�ⷴӦ����![]() ��
��![]() �ữ����
�ữ����![]() ��
��![]() ������������Ӧ����
������������Ӧ����![]() ���ϳ�·��Ϊ��CH3COOH
���ϳ�·��Ϊ��CH3COOH![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() ��
��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ������ʵ�������ȷ���ܴﵽ��ӦĿ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ��ȡ2.0gNaOH���� | ���������ϸ���1����ֽ��Ȼ��������������2g���룬����������NaOH���� |
B | ����ϡ���� | �Ƚ�Ũ��������ձ�����������ˮ |
C | ��֤����������ʴ | �����������Թ��У��������û |
D | ������Һ���Ƿ���NH4+ | ȡ������Һ���Թ��У�����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ������������� |
A. A B. B C. C D. D
����Ŀ��C��N��O��Na��Mg��Al��S��Cl�dz����İ���Ԫ�أ�����Ԫ�ؼ��仯�������ʣ��ش���������:
��1��S�����ڱ��е�λ��Ϊ______��CO2�ĵ���ʽ��_______��
��2���Ƚ�O��NaԪ�س������ӵİ뾶��С(�û�ѧʽ��ʾ����ͬ)____>____��__________�Ƚ�S��ClԪ�ص�����������Ӧˮ���������ǿ��:____>____ ��_______________
��3����֪:
������ | MgO | Al2O3 | MgCl2 | AlCl3 |
���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
�۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����___________������ʱ�����Al2O3�������AlCl3��ԭ����___________ ��
��4��̼��þ�γɵ�1mol������Q��ˮ��Ӧ������2molMg(OH)2��1mol��������������̼��������Ϊ9:1,���Ľṹ��ʽΪ______��Q�Ļ�ѧʽΪ_______ ��
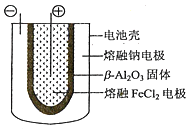
��5������״̬�£��ƺ�FeCl2����ɿɳ����(װ��ʾ��ͼ����)����Ӧԭ��Ϊ:2Na+FeCl2![]() Fe+2NaCl.�ŵ�ʱ����ص�������ӦʽΪ___________�����ʱ��_______(д��������)�缫�ӵ�Դ�ĸ������õ�صĵ����Ϊ_______��
Fe+2NaCl.�ŵ�ʱ����ص�������ӦʽΪ___________�����ʱ��_______(д��������)�缫�ӵ�Դ�ĸ������õ�صĵ����Ϊ_______��