��Ŀ����
(5��)����һ�ֵ����Ϻ����ḻ��Ԫ�أ������仯������о���������������������Ҫ���塣
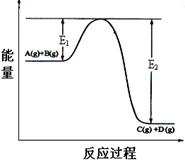
��1����ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ
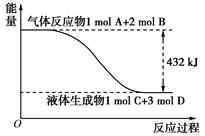
(2) ��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ�������������
��ش��������⣺
��1����֪����N2(g)+O2(g)=2NO(g) ��H1= +180.5kJ/mol
��N2(g)+3H2(g) 2NH3(g) ��H2=��92.4kJ/mol
2NH3(g) ��H2=��92.4kJ/mol
��2H2(g)+O2(g)=2H2O(g) ��H3=��483.6kJ/mol
д������������������һ�����������ˮ�������Ȼ�ѧ����ʽ��
��1����ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ

(2) ��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ�������������
��ش��������⣺
��1����֪����N2(g)+O2(g)=2NO(g) ��H1= +180.5kJ/mol
��N2(g)+3H2(g)
 2NH3(g) ��H2=��92.4kJ/mol
2NH3(g) ��H2=��92.4kJ/mol��2H2(g)+O2(g)=2H2O(g) ��H3=��483.6kJ/mol
д������������������һ�����������ˮ�������Ȼ�ѧ����ʽ��
NO2(g)+CO(g)=CO2(g)+NO(g) ��H=" ��234" mol��L-1 ��2�֣�
4NH3(g) +5 O2(g) ="4NO(g)" + 6H2O(g) ��H=" ��905.0" mol��L-1��3�֣�
4NH3(g) +5 O2(g) ="4NO(g)" + 6H2O(g) ��H=" ��905.0" mol��L-1��3�֣�
��1����ͼ���֪����H= ��|E1��E2|=" ��234" mol��L-1
��2�����ݸ�˹���ɿ�֪���١�2���ڡ�2 + 3��3������ô𰸡�
��2�����ݸ�˹���ɿ�֪���١�2���ڡ�2 + 3��3������ô𰸡�

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
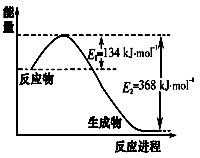
 2NH3(g)��H= -38.6kJ��mol-1
2NH3(g)��H= -38.6kJ��mol-1 C(g)+D(g) ��H =" Q" kJ/mol
C(g)+D(g) ��H =" Q" kJ/mol