��Ŀ����
4����ʵ��ȷ��ij��HA��������ʣ���ͬѧ�ķ����ǣ������ף��ٳ�ȡһ��������HA����0.1mol/L����Һ100mL��
����pH��ֽ�������Һ��pH������֤��HA��������ʣ�
�ң�������֪���ʵ���Ũ�ȵ�HA��Һ�����ᣬ�ֱ�����pH=1����������Һ��100mL��
�ڷֱ�ȡ��������Һ��10mL����ˮϡ��Ϊ100mL��
�۸�ȡ��ͬ���������ϡ��Һװ�������Թܣ�ͬʱ���봿����ͬ��п�����۲�������֤��HA��������ʣ�
��1�������������ĵڢٲ��У���Ҫ�õ��Ķ���������100mL����ƿ��
��2����pH��ֽ�ⶨHA��ҺpH�IJ�������Ϊ��һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����գ�
��3�������У�˵��HA��������ʵ������Dz����Һ��pH��1��ѡ�������=����
��4���ҷ����У�˵��HA��������ʵ�������b��ѡ���ţ���
��a��װHCl��Һ���Թ��зų�H2�����ʿ죻
��b��װHA��Һ���Թ��зų�H2�����ʿ죻
��c�������Թ��в�����������һ���죮
���� ��1����������������ѡȡ������
��2���õ�����Һ����ֽ�����ɫ���Աȣ�
��3��������ʲ��ֵ��룬���ڵ���ƽ�⣬��ˮϡ�ͣ��ٽ�����ĵ��룻
��4��������ʲ��ֵ��룬��Ӧ������������Ũ�ȳ����ȣ�pH��ȵ�ǿ�������ϡ����ͬ�ı�����������������Ũ�ȴ���ǿ�ᣮ
��� �⣺��1�������������ĵڢٲ��У���Ҫ�õ��Ķ���������100mL����ƿ���ʴ�Ϊ��100mL����ƿ��
��2��pH��ֽ�ⶨ��ҺpH�IJ����ǣ���һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����գ�
�ʴ�Ϊ����һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����գ�
��3��������ʲ��ֵ��룬��0.1mol/L����Һ��������Ũ��С��0.1mol/L������pH��1���ʴ�Ϊ������
��4��pH��ȵ�ǿ�����У���ˮϡ�ʹٽ�������룬����ϡ�ͺ�������������Ũ�ȴ���ǿ�ᣬ������Ũ��Խ��Ӧ����Խ���������װHA��Һ���Թ��зų�H2�����ʿ��֤��HAΪ���ᣬ��ѡb��
�ʴ�Ϊ��b��
���� ������һ���йص���ʵĵ���֪ʶ���ۺ�Ӧ���⣬����ѧ�������ͽ��������������ѶȲ���

 ��������������������ϵ�д�
��������������������ϵ�д�| A�� | һ������BaSO4���� | B�� | һ������BaSO3���� | ||
| C�� | һ��û��SO2�ݳ� | D�� | һ����SO3�ݳ� |
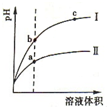 ij�¶��£���ͬ�������ͬpH������ʹ�����Һ�ֱ��ˮϡ�ͣ���Һ��pH����Һ����仯��������ͼ��ʾ�������ж���ȷ���ǣ�������
ij�¶��£���ͬ�������ͬpH������ʹ�����Һ�ֱ��ˮϡ�ͣ���Һ��pH����Һ����仯��������ͼ��ʾ�������ж���ȷ���ǣ�������| A�� | ��Ϊ����ϡ��ʱ��pH�仯���� | |
| B�� | a��Kw����ֵ��c��Kw����ֵ�� | |
| C�� | b�������Ũ�ȴ���a�������Ũ�� | |
| D�� | b����Һ�ĵ����Ա�c����Һ�ĵ�����ǿ |
| A�� | �����Һ�����NaOH��Һ���������ɫ�����������Һ�п϶�����Fe3+ | |
| B�� | �����Һ�����BaCl2��Һ����������ɫ�������ټ������ϡ�����ɫ�������ܽ⣬���������Һ�п϶�����SO42- | |
| C�� | �����Һ�������������������ټ�����������Һ�����������ɣ����������Һ�п϶�����Cl- | |
| D�� | �����Һ�����CaCl2��Һ����������ɫ����������ϡ���������ɫ��ζ���壬������ͨ�����ʯ��ˮ�У���Һ����ǣ����������Һ�п϶���HCO3- |
| ѡ�� | H2SO4 | Na2S2O3 | H2O |
| A | 5mL0.1mol/L | 5mL0.1mol/L | 0 |
| B | 5mL0.2mol/L | 5mL0.1mol/L | 10 |
| C | 5mL0.3mol/L | 5mL0.1mol/L | 20 |
| D | 5mL0.4mol/L | 5mL0.1mol/L | 30 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Cl2ͨ��NaOH��Һ�� Cl2+2OH-�TCl-+ClO-+H2O | |
| B�� | ��������ϡ���ᷴӦ FeS+2H+�TFe2++H2S�� | |
| C�� | ����狀�����������Һ Ba2++SO42-�TBaSO4�� | |
| D�� | ����������ϡ���ᷴӦ Ba2++SO42-+2H++2OH-�TBaSO4��+2H2O |
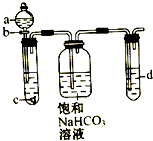 ijͬѧΪ��̽��Ԫ�طǽ����Եݱ���ɣ��������ͼ��ʾʵ��װ�ã�
ijͬѧΪ��̽��Ԫ�طǽ����Եݱ���ɣ��������ͼ��ʾʵ��װ�ã�