��Ŀ����
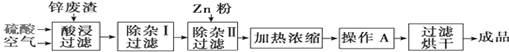
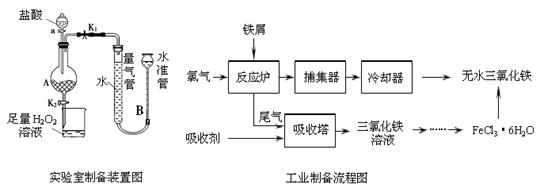
�����������ѧ���õ��Լ�����ҵ�������̿��Ʊ���������������¡�
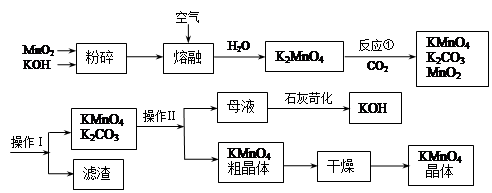
��1��д��ʵ��������KMnO4�ֽ���ȡO2�Ļ�ѧ����ʽ
��2��KMnO4ϡ��Һ��һ�ֳ��õ���������������ԭ��������������ͬ����
��3���ڵζ�ʵ���У����� �����ʽ����ʽ�����ζ�����ȡKMnO4��Һ��
��4��д����Ӧ�ٵĻ�ѧ����ʽ
��5��������������� �����������KMnO4��K2CO3�������� ����
���ʣ��ϵIJ��죬���� ����������裩�����ȹ��˵õ�KMnO4�־��塣
��6�����������п���ѭ��ʹ�õ������� �� ��д��ѧʽ��,���ڴ�����100�����̿�MnO287.0%���������Ͽ�����KMnO4���� �֣��������Ʊ�������ԭ�ϵ���ʧ����

��1��д��ʵ��������KMnO4�ֽ���ȡO2�Ļ�ѧ����ʽ
��2��KMnO4ϡ��Һ��һ�ֳ��õ���������������ԭ��������������ͬ����
| A��84����Һ(NaClO��Һ) |
| B��˫��ˮ |
| C������ |
| D��75%�ƾ� |
��4��д����Ӧ�ٵĻ�ѧ����ʽ
��5��������������� �����������KMnO4��K2CO3�������� ����
���ʣ��ϵIJ��죬���� ����������裩�����ȹ��˵õ�KMnO4�־��塣
��6�����������п���ѭ��ʹ�õ������� �� ��д��ѧʽ��,���ڴ�����100�����̿�MnO287.0%���������Ͽ�����KMnO4���� �֣��������Ʊ�������ԭ�ϵ���ʧ����
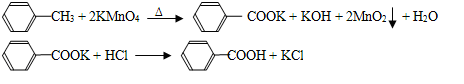
��1��2KMnO4 K2MnO4��MnO2��O2�� ��2�֣�
K2MnO4��MnO2��O2�� ��2�֣�
��2��AB ����1�֣���2�֣�ѡ�����÷֣�
��3����ʽ ��2�֣�
��4��3K2MnO4��2CO2 = 2KMnO4��2K2CO3��MnO2��2�֣�
��5�����ˣ�1�֣����ܽ�ȣ�1�֣���Ũ���ᾧ��1�֣�
��6��MnO2��1�֣���KOH��1�֣� 158��2�֣�
 K2MnO4��MnO2��O2�� ��2�֣�
K2MnO4��MnO2��O2�� ��2�֣���2��AB ����1�֣���2�֣�ѡ�����÷֣�
��3����ʽ ��2�֣�
��4��3K2MnO4��2CO2 = 2KMnO4��2K2CO3��MnO2��2�֣�
��5�����ˣ�1�֣����ܽ�ȣ�1�֣���Ũ���ᾧ��1�֣�
��6��MnO2��1�֣���KOH��1�֣� 158��2�֣�
�����������1��ʵ��������KMnO4�ֽ���ȡO2�Ļ�ѧ����ʽ2KMnO4
 K2MnO4��MnO2��O2��
K2MnO4��MnO2��O2����2��KMnO4ϡ��Һ��һ�ֳ��õ���������������ԭ��������������ǿ�����ԡ�����������������ԭ����ͬ����A��84����Һ�������˴������ǿ�����ԣ���ȷ��B��˫��ˮ����ǿ�����ԣ���ȷ��C��������������������ʹϸ�������ʱ��ԣ�����D��75%�ƾ���������������ʹϸ�������ʱ��ԣ�����ѡAB��
��3����Ϊ������ؾ���ǿ�����ԣ���������ʽ�ζ�����ȡ��
��4�������������д����Ӧ�ٵĻ�ѧ����ʽ3K2MnO4��2CO2 = 2KMnO4��2K2CO3��MnO2
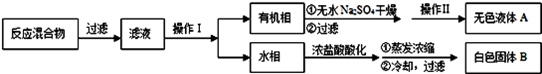
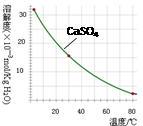
��5������Һ�������룬�ù��˵ķ��������Բ���1Ϊ���ˣ�KMnO4��K2CO3���������ܽ���ϲ������K2CO3���ܽ�ȸ������������ܽ�ȵIJ�ͬ������Ũ���ᾧ�����ȹ��˵õ�������ؽ��壻
��6��������ͼ�пɿ�����MnO2��KOH��ʼ���ģ��������ɣ����Կ�ѭ��ʹ�ã�����ѭ��ʹ�ã�����Ϊ��������ȫ��ת��Ϊ������أ���MnO2��KMnO4�����̿���MnO2��������87�֣����Կ�����KMnO4��������158�֡�

��ϰ��ϵ�д�
�����Ŀ
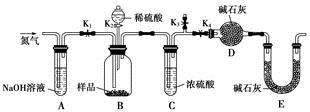
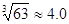 ��
�� 2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4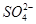 ��2H2O
��2H2O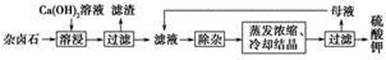
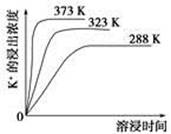
 ?
? CaCO3(s)��
CaCO3(s)��