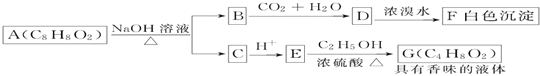
��Ŀ����
����Ŀ��һ�������£�ͨ�����з�Ӧ��ʵ��ȼú��������Ļ��գ�SO2(g)��2CO(g)![]() 2CO2(g)��S(l)����H<0������Ӧ�ں��ݵ��ܱ������н��У������й�˵����ȷ����(����)
2CO2(g)��S(l)����H<0������Ӧ�ں��ݵ��ܱ������н��У������й�˵����ȷ����(����)
A. ƽ��ǰ�����ŷ�Ӧ�Ľ��У�������ѹǿʼ�ղ���
B. ƽ��ʱ�������������䣬�����������Ӧ���ʼӿ�
C. ƽ��ʱ�������������䣬�����¶ȿ����SO2��ת����
D. �����������䣬ʹ�ò�ͬ�������÷�Ӧ��ƽ�ⳣ������
���𰸡�D
�����������������A��SΪҺ�壬��Ӧǰ������ϵ��֮�Ͳ���ȣ����ŷ�Ӧ���У��������ʵ�����С��ѹǿ��С���ʴ���B����ΪSΪҺ�壬Ũ����Ϊ��������˷����S����Ӧ���ʲ��䣬�ʴ���C���˷�Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ���SO2��ת���ʽ��ͣ��ʴ���D����ѧƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬�¶Ȳ��䣬��ѧƽ�ⳣ�����䣬����ȷ��

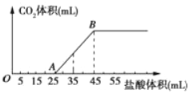
����Ŀ��I��25��ʱ����25ml�������Ʊ���Һ����μ���0.2 mol��L��1��һԪ��HA��Һ��pH�仯��������ͼ��ʾ��

��1��������������Һ�����ʵ���Ũ��Ϊ________mol��L��1��
��2��A���Ӧ������Ϊ12.5ml�������û����Һ����ˮ�������c(OH��)��_______ mol��L��1��
��3��HA��________�ᣨѡ�ǿ��������������NaA��ˮ��Һ��________�ԣ�ѡ��ᡱ���������ԭ���ǣ�________�������ӷ���ʽ��ʾ����
��4����B�����û����Һ�У�c(Na��) ��c(A��)��c(H��)��c(OH��)�ɴ�С��˳����_________��
��5����C�����û����Һ�У�����˵��ȷ���ǣ�___________��
A��HA�ĵ���̶�С��NaA��ˮ��̶�
B������Ũ���ɴ�С��˳����c(Na��) ��c(A��)��c(H��)�� c(OH��)��
C��c(Na+) ��c(H+)��c(A��) ��c(OH��)
D��c(HA)��c(A��)��0.2mol��L��1
����25 ��������£�ijЩ����ĵ���ƽ�ⳣ����
��ѧʽ | CH3COOH | HClO | H2CO3 | H2C2O4 |
Ka | Ka��1.8��10��5 | Ka��3.0��10��8 | Ka1��4.1��10��7 Ka2��5.6��10��11 | Ka1��5.9��10��2 Ka2��6.4��10��5 |
��1���¶ȡ�Ũ����ͬ��CH3COOH��HClO��Һ��ˮ�ĵ���̶�ǰ��________ ���ߣ��>����=����<������
��2����ͬ�¶��£�pH��ͬ��NaClO��CH3COOK������Һ�У�c(CH3COOK)________c(NaClO) ���>����=����<������[c(Na��)��c(ClO��)]_______[c(K��)��c(CH3COO��)]���>����=����<������
��3����25 ��������£���0.1 mol��L��1 CH3COOH��Һ�еμ�NaOH��Һ��c(CH3COOH)��c(CH3COO��)��5��9����ʱ��ҺpH��________��