��Ŀ����
����Ŀ��(1)���ڷ�Ӧ��2NO(g)��O2(g)![]() 2NO2(g)��������������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ(p1��p2)���¶ȱ仯������(��ͼ)��
2NO2(g)��������������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ(p1��p2)���¶ȱ仯������(��ͼ)��
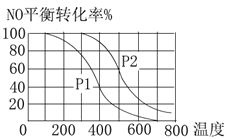
�ٱȽ�p1��p2�Ĵ�С��ϵ��________��
�����¶����ߣ��÷�Ӧƽ�ⳣ���仯��������________(����������������С��)��
(2)���ݻ�Ϊ1.00 L�������У�ͨ��һ������N2O4��������ӦN2O4(g)![]() 2NO2(g)����֪���¶����ߣ��˻���������ɫ���
2NO2(g)����֪���¶����ߣ��˻���������ɫ���
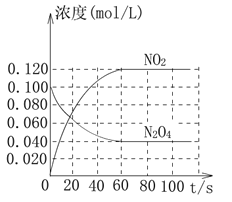
�ش��������⣺
�ٷ�Ӧ����H_________________________(��������������С����)0��100 ��ʱ����ϵ�и�����Ũ����ʱ��仯����ͼ��ʾ����0��60sʱ�Σ���Ӧ����v(N2O4)Ϊ____________mol��L��1��s��1����Ӧ��ƽ�ⳣ��K1Ϊ____________________��
��100 ��ʱ��ƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.002 0 mol��L��1��s��1��ƽ�����ʽ��ͣ���10 s�ִﵽƽ�⡣��ʽ�����¶�Tʱ��Ӧ��ƽ�ⳣ��K2=_________________��
���¶�Tʱ��Ӧ��ƽ�����Ӧ�������ݻ�����һ�룬ƽ����________________(��������Ӧ�������淴Ӧ��)�����ƶ����ж�������__________________________��
���𰸡� p2>p1 ��С ���� 0.001 0 0.36 ƽ��ʱ��c(NO2)��0.120 mol��L��1��0.002 0 mol��L��1��s��1��10 s��2��0.160 mol��L��1��c(N2O4)��0.040 mol��L��1��0.002 0 mol��L��1��s��1��10 s��0.020 mol��L��1��K2��![]() ��1.28 �淴Ӧ ����Ӧ�����������Сһ�룬������ѹǿ����������������ʱ������ѹǿ��ƽ�����������ʻ�ѧ��������С�ķ����ƶ��������淴Ӧ�����ƶ�
��1.28 �淴Ӧ ����Ӧ�����������Сһ�룬������ѹǿ����������������ʱ������ѹǿ��ƽ�����������ʻ�ѧ��������С�ķ����ƶ��������淴Ӧ�����ƶ�
��������(1)����֪2NO(g)+O2(g)2NO2(g)�������������С�ķ�Ӧ������ѹǿƽ�����ƣ���NO��ת���ʻ�������ͼ��֪p2ʱNO��ת���ʴ���p2ʱѹǿ��p2>p1���ʴ�Ϊ��p2>p1��
����ͼ���֪�������¶ȵ����ߣ�NO��ת���ʼ�С��˵�������¶�ƽ�����ƣ���÷�Ӧ�������Ƿ��ȷ�Ӧ�����������¶�ƽ�ⳣ��K��С���ʴ�Ϊ����С��
(2)�����¶ȵ����ߣ�����������ɫ�����ѧƽ��������Ӧ�����ƶ�������H��0��0��60sʱ�Σ�N2O4Ũ�ȱ仯Ϊ��0.1mol/L-0.04mol/L=0.06mol/L��v(N2O4)= ![]() =0.0010molL-1s-1��K=
=0.0010molL-1s-1��K= =
=![]() =0.36���ʴ�Ϊ������0.0010molL-1s-1��0.36��
=0.36���ʴ�Ϊ������0.0010molL-1s-1��0.36��
��ƽ��ʱ��c(NO2)��0.120 mol��L��1��0.002 0 mol��L��1��s��1��10 s��2��0.160 mol��L��1��c(N2O4)��0.040 mol��L��1��0.002 0 mol��L��1��s��1��10 s��0.020 mol��L��1��K2��![]() ��1.28���ʴ�Ϊ��
��1.28���ʴ�Ϊ��![]() ��1.28��
��1.28��
�۷�Ӧ�������ݻ�����һ�룬ѹǿ��������Ӧ�������������������ѹǿ�������������С�ķ����ƶ����ʴ�Ϊ���淴Ӧ������Ӧ�����������Сһ�룬������ѹǿ����������������ʱ������ѹǿ��ƽ�����������ʻ�ѧ��������С�ķ����ƶ��������淴Ӧ�����ƶ���

����Ŀ���л���A���������Ƿ��͵õ�,Ҳ�ɴ���ţ������ȡ��������AΪ��ɫ��Һ��,������ˮ��Ϊ�о�A�������ṹ,����������ʵ��:
ʵ�鲽�� | ���ͻ�ʵ����� |
��1����ȡA 9.0 g,����ʹ������,�����ܶ�����ͬ������H2��45�� | ��ͨ���������: (1)A����Է�������Ϊ____ |
��2����9.0 g A��������O2�г��ȼ��,��ʹ��������λ���ͨ��Ũ���ᡢ��ʯ��,�������߷ֱ�����5.4 g��13.2 g | (2)A�ķ���ʽΪ_____ |
��3����ȡA 9.0 g,��������NaHCO3��ĩ��Ӧ,����2.24 L CO2(��״��),�������������Ʒ�Ӧ������2.24 L H2(��״��) | (3)�ýṹ��ʽ��ʾA�к��еĹ�����:___________________________ |
��4��A�ĺ˴Ź���������ͼ:
| (4)A�к���____����ԭ�� |
��5����������,A�Ľṹ��ʽΪ______����д��A��NaHCO3��Ӧ�Ļ�ѧ����ʽ______ | |

