��Ŀ����
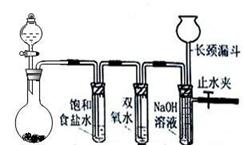
��12�֣���Ȼˮ����������ˮ����Ҫ��Դ������Ȼˮ��ÿ������õ�ˮһ���뾭�����������ɱ�������Ȳ��衣
��1������������Ҫ��ˮ�м������������罫���μ���ˮ���ܴﵽ��ˮĿ�ģ�
ԭ���� �������ӷ���ʽ��ʾ����
��2����������������ˮɱ����������������ӷ���ʽ���������� ��
��3������ˮ������������� (K2FeO4)����ǿ���������ú��������á���ҵ�Ͽ�ͨ�����������Ʊ�������أ�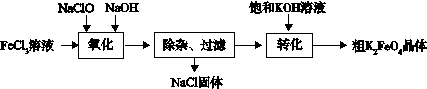
��������:�������������Ի�������Һ�л��ֽ⣬�ڼ�����Һ���ȶ���
��ɡ������������е����ӷ���ʽ
��Fe3+ + ��ClO- +�� ="��" FeO42- + ��Cl- + ��
��ת����������ʵ����Na2FeO4�Ƶ�K2FeO4�������ö��� �ԵIJ�ͬ��
�۽��������ɴ�K2FeO4������ᴿ�����ֲ�Ʒ�� �ܽ⣬Ȼ���ټ��뱥��KOH��Һ����ȴ�ᾧ�����ˡ�
�ܸ�����ص�Ӧ�û��ڲ�����չ�С�����Ƴɸ�����أ� ��ط�ӦΪ��
3Zn + 2K2FeO4 + 8H2O  3Zn(OH)2 + 2Fe(OH)3 + 4KOH
3Zn(OH)2 + 2Fe(OH)3 + 4KOH
�ŵ�ʱ��������ӦΪ�� ��
��12�֣�
��1��Al3++3H2O Al(OH)3�����壩+ 3 H+ ��2�֣�
Al(OH)3�����壩+ 3 H+ ��2�֣�
��2�� Cl2 + H2O =" HCl" + HClO�����ɵ�HClO��ǿ�����ԣ�����ɱ���������á�
��2�֣�
��3���� 2Fe3+ + 3ClO- + 10 OH- = 2FeO42- + 3Cl- +5H2O ��2�֣�
���ܽ� ��2�֣�
��ϡKOH��Һ�� ��2�֣�
�� 2FeO42-+6e-+8H2O==2Fe(OH)3+10OH- ��2�֣�
���������������1�����μ���ˮ���ܴﵽ��ˮĿ�ģ�����Ϊ������ˮ��������������壬�����������ã����ӷ���ʽ��Al3++3H2O Al(OH)3�����壩+ 3 H+
Al(OH)3�����壩+ 3 H+
��2����������ˮ���ɴ����ᣬCl2 + H2O =" HCl" + HClO�����������ǿ�����ԣ�����ɱ��������
��3���ٸ�����Ŀ������ͼ�жϷ�Ӧ�������������ƣ����Է�Ӧ��Ŀհ״�Ӧ��OH-�������Ŀհ״�Ӧ��H2O�����ݵ�ʧ�����غ㣬��ƽ�û�ѧ����ʽ������2Fe3+ + 3ClO- + 10 OH- = 2FeO42- + 3Cl- +5H2O ��
�ڡ�ת����������ʵ����Na2FeO4�Ƶ�K2FeO4�������ö����ܽ��ԵIJ�ͬ��K2FeO4��Na2FeO4���ܽ��С��
�۸������������Ի�������Һ�л��ֽ⣬�ڼ�����Һ���ȶ������Խ��ֲ�Ʒ��ϡKOH��Һ�ܽ⣻
�ܷŵ�ʱ������ѧ��ת��Ϊ���ܣ�����������ԭ��Ӧ���缫����ʽΪ2FeO42-+6e-+8H2O==2Fe(OH)3+10OH-
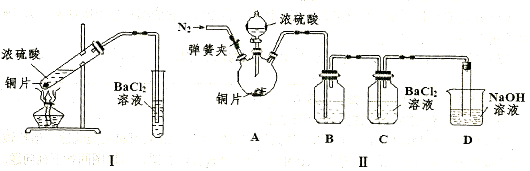
���㣺�������ӵ�ˮ�⣬��������;����ѧ����ʽ����ƽ���绯ѧԭ����Ӧ��

 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�п������һ�ְ�ɫ���ϡ���ҵ������ZnSO4��BaS��Һ��϶��ɣ�BaS+ZnSO4=ZnS��+BaSO4������������¹�ҵ�������̻ش��й����⡣
I��ZnSO4��Һ���Ʊ����ᴿ��
�й����ϣ�a����п�����Ҫ�ɷ���ZnCO3��������SiO2��FeCO3��Cu2(OH)2CO3�ȣ�b��Zn(OH)2��Al(OH)3���ƣ������ڹ�����NaOH��Һ����Na2ZnO2��
��1������1�Ļ�ѧʽΪ ������ʹ�õ���������������е� ������ţ���
| A��Cl2 | B��H2O2 | C��KMnO4 | D��ŨHNO3 |
��3������ܷ��������ӷ�Ӧ����ʽΪ ��
II��BaS��Һ���Ʊ�

�й����ݣ�Ba��s����S��s����2O2��g����BaSO4��s�� ��H1 = ��1473.2 kJ?mol��1
C��s���� 1/2O2��g����CO��g�� ��H2 = ��110.5 kJ?mol��1
Ba��s���� S��s����BaS��s�� ��H3 = ��460 kJ?mol��1
��4�������ջ�ԭ�IJ����ΪBaS��CO�����䷴Ӧ���Ȼ�ѧ����ʽΪ��
��
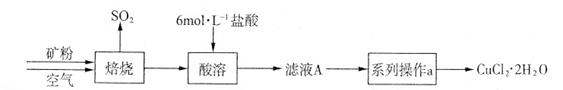
��.��ȡп����
��5��������в����ʹ����������������ĺ���� ��
��12�֣�������ȷֽ���ʵ������ȡ������һ�ַ�����ij̽��С��������ϵ�֪��������ڲ�ͬ�����·ֽ������£�
| ʵ�� | ��Ӧ��ϵ | ��һ���ȷ��¶ȣ��棩 | �ڶ����ȷ��¶ȣ��棩 |
| l | KC1O3 | 400��������ų��� | 480������������ų��� |
| 2 | KC1O3+MnO2 | 350���д�������ų��� | |
 KCl+2O2������д����400��Ļ�ѧ��Ӧ����ʽ ����ʾ����Ӧ��ֻ����Ԫ�صĻ��ϼ۸ı䣩��
KCl+2O2������д����400��Ļ�ѧ��Ӧ����ʽ ����ʾ����Ӧ��ֻ����Ԫ�صĻ��ϼ۸ı䣩����С��ͬѧ����ʵ��2����ȡ����ʱ����ʵ���з������ɵ������Դ���ɫ�����д̼�����ζ��
��������⡿ʵ�������ɵ������г������������ʲô���ʣ�
���������ϡ�1���������ȳ������ǻ�ɫ��ǿ�Ҵ̼�����ζ������
2���������Ⱦ���ǿ�����ԣ�����Һ���ܹ��Ѷ������������ļ��̣�ʹ֮�γɲ�����ˮ�Ķ������̣�2ClO2+5Mn2++6H2O��5MnO2��+12H++2Cl���������������ܡ�
��������衿����l����������������2�����ж������ȡ�
�����ʵ�顿��ͬѧ���ʵ����֤����1������
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ������������Ӵ�ʪ��ĵ��۵⻯����ֽ | | ����l��ȷ |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| | | ����2��ȷ |

 CuCl42-(��ɫ)+4H2O��
CuCl42-(��ɫ)+4H2O��




 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O