��Ŀ����
��ҵ�ϣ�����ͭ��CuFeS2��ͨ��8CuFeS2+21O2 8Cu+4FeO+2Fe2O3+16SO2��Ӧ��ȡͭ����������Ļ����
8Cu+4FeO+2Fe2O3+16SO2��Ӧ��ȡͭ����������Ļ����
��1��������Ӧ�У���ԭ��Ϊ ��
��2����ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3��
��������Ϣ�ش��������⣺
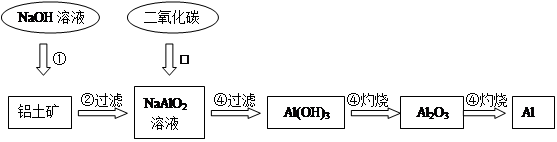
a��ͨ�������ڣ�¯���е�Al2O3����� ��д���ӣ���
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ ����KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ ��
֤��¯���к���FeO��ʵ������Ϊ ��
��3��������¯���н��к������IJⶨ�������£�
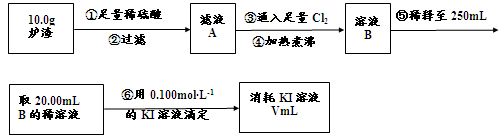
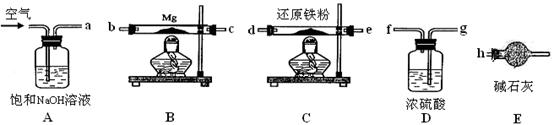
I������۷�����Ӧ�����ӷ���ʽΪ ��
II����������������� ��
III����������õ��IJ����������ձ�������������ͷ�ιܡ� ��
IV�����ζ�����������0.100mol��L?1KI��Һ20.00mL����¯�������İٷֺ���Ϊ ��
 8Cu+4FeO+2Fe2O3+16SO2��Ӧ��ȡͭ����������Ļ����
8Cu+4FeO+2Fe2O3+16SO2��Ӧ��ȡͭ����������Ļ������1��������Ӧ�У���ԭ��Ϊ ��
��2����ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3��
��������Ϣ�ش��������⣺
a��ͨ�������ڣ�¯���е�Al2O3����� ��д���ӣ���
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ ����KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ ��
֤��¯���к���FeO��ʵ������Ϊ ��
��3��������¯���н��к������IJⶨ�������£�

I������۷�����Ӧ�����ӷ���ʽΪ ��
II����������������� ��
III����������õ��IJ����������ձ�������������ͷ�ιܡ� ��
IV�����ζ�����������0.100mol��L?1KI��Һ20.00mL����¯�������İٷֺ���Ϊ ��
��1��CuFeS2 ��2��a��[Al(OH)4]-��AlO2- b��ϡ���ᡢ����KMnO4��Һ��ϡ�����ȡ¯��������Һʹ����KMnO4��Һ��ɫ��3��I��2Fe2++Cl2= 2Fe3++2Cl-II��������Һ���ܽ�Ĺ�����Cl2 III��250mL����ƿ ��δ��250mL�������֣�IV��14%
�����������1���ڷ�Ӧ��CuFeS2����Ԫ�صĻ��ϼ���+2�۱�Ϊ+3�ۣ���Ԫ�صĻ��ϼ���-2�۱�Ϊ+4������ԭ������2������ϡ�����ȡ¯�������ķ�ӦΪ��Al2O3 + 6H+=2Al3+ + 3H2O
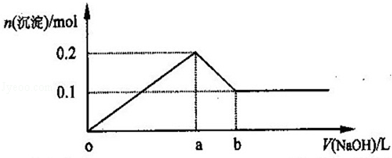
FeO + 2H+ = Fe2+ + H2O Fe2O3 + 6H+ = 2Fe3+ + 3H2O ����Һ�к��еĽ�����������Al3+��Fe2+��Fe3+����������Al3+��Fe3+���ӹ�������������Һ�����ķ�Ӧ��Al3+ + 4OH-=AlO2- + 2H2O Fe3+ + 3OH- = Fe(OH)3�� a��ͨ�������ڣ�¯���е�Al2O3�����AlO2-��b��֤¯���к���+2�۵�����Ӧ�ȼ���ϡ�����ܽ⣬�����������ӣ����л�ԭ�ԣ����������ط���������ԭ��Ӧ���ữʱ���ܼ������ᣬ�����������ط���������ԭ��Ӧ��Ҳ����ֱ�Ӽ������ᣬ�������������ӣ����ܼ��飬�ʴ�Ϊ��ϡ���ᡢKMnO4��Һ��ϡ�����ȡ¯��������Һ�����������ӣ��������ط���������ԭ��Ӧ��ʹ���������ɫ���ʴ�Ϊ��ϡ�����ȡ¯��������Һ��ʹKMnO4��Һ��ɫ����3��������������ͼ�����ķ�ӦΪ��¯��������ϡ���Al2O3 + 6H+=2Al3+ + 3H2O FeO + 2H+ = Fe2+ + H2O Fe2O3 + 6H+ = 2Fe3+ + 3H2O���ˣ���Һ�к��еĽ�����������Al3+��Fe2+��Fe3+��ͨ������Cl2��2Fe2+ + Cl2 = 2Fe3+ + 2Cl- �ζ�ԭ����2Fe3+ + 2I- = 2Fe2+ + I2I�������ͨ����������������Ӧ�����ӷ���ʽΪ2Fe2+ + Cl2 = 2Fe3+ + 2Cl- ��II������������������I-����Ӱ��ʵ���������Բ��������е������Ǹ�����Һ���ܽ�Ĺ�����Cl2����III�������Ϊ��Һ�����ƣ��õ��IJ����������ձ�������������ͷ�ιܡ�250mL����ƿ��IV���ζ������з����ķ�ӦΪ2Fe3+ + 2I- = 2Fe2+ + I2���ɷ�Ӧʽ֪n(I-)= n(Fe3+)=0.002mol���������ͼ֪10.0g¯�����������ʵ���Ϊ0.025mol������Ϊ1.4g,��������Ϊ14%��

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ


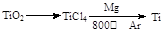
 MgO+H2�� Mg3N2 +6H2O =3Mg(OH)2+2NH3��
MgO+H2�� Mg3N2 +6H2O =3Mg(OH)2+2NH3��