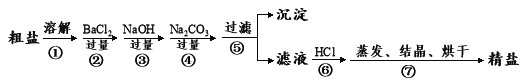
��Ŀ����
����Ŀ������ڹ�ũҵ������������Ҫ����;�������Ҫ��������и��⡣
I.ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ��NH4��2SO4��Һ��

��1����װ��A�Ʊ�����ʱ������a���Ƿ��а��������ķ�����____________��
��2����ȡ��NH4��2SO4��Һʱ�������ӵ�˳�����ýӿ������ĸ��ʾ���ǣ�a �� _____________��
��3����װ��C������Һ���������IJ��������� ��
��.Ϊ����Ȼ�淋ľ��ü�ֵ���ҹ���ѧ�����������������þ�ȷֽ��Ȼ���ư������õ���ʽ�Ȼ�þ[Mg��OH��Cl]�Ĺ��ա�ijͬѧ���ݸ�ԭ����Ƶ�ʵ��װ����ͼ��
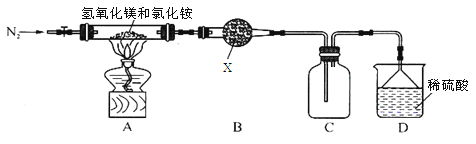
��4��װ��A�з�����Ӧ���ɼ�ʽ�Ȼ�þ�Ļ�ѧ����ʽΪ ��
��5��װ��B���Լ�XΪ________________��
��6������װ��ͼ�е�һ��������______________��
��7��MgCl2��6H2O�ڿ����м��ȣ�����Mg��OH��Cl������MgCl2��6H2O�Ʊ���ˮMgCl2��Ӧ_________��
���𰸡�
��1����ʪ��ĺ�ɫʯ����ֽ����a�ڴ�������ֽ��������˵���а���������
��2��d e f��
��3����Һ©����
��4��Mg��OH��2+NH4Cl![]() Mg��OH��Cl+NH3��+H2O��
Mg��OH��Cl+NH3��+H2O��
��5����ʯ����
��6��C�е�����Ӧ�̽�������
��7����HCl���������
��������
�����������1�������Լ��ԣ�����a���Ƿ��а��������ķ�������ʪ��ĺ�ɫʯ����ֽ����a�ڴ�������ֽ��������˵���а����������ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ����a�ڴ�������ֽ��������˵���а���������
��2��������������ˮ��������������������ͨ��C�а��������ᷴӦ��������泥�ע��Ӧ�����̳���ֹ�����������İ�����Dװ���������ʴ�Ϊ��d e f��
��3��ˮ�����Ȼ�̼�������ܣ��ܶȲ�ͬ�������������ˮ������ͨ����Һ�ķ���������ߣ�ʹ�õIJ��������Ƿ�Һ©�����ʴ�Ϊ����Һ©����
��.��װ������ͨ�뵪�����ų�װ������������A���Ȼ�狀�������þ�������ֽⷴӦ���ɼ�ʽ�Ȼ�þ[Mg��OH��Cl]��ͬʱ���ɰ�����ˮ����Ӧ����ʽΪMg��OH��2+NH4ClMgOHCl+NH3��+H2O��
��ʯ��������ˮ���Ӷ������������ʯ�ҺͰ�������Ӧ�����Լ�ʯ���ܸ��ﰱ����
������������ˮ����һˮ�ϰ���һˮ�ϰ����Ȼ����������ֽⷴӦ������������������ͬʱ�����Ȼ�泥�������������ˮ����ˮ��Һ�ʼ��ԣ������ܺ�ϡ���ᷴӦ��������泥����Կ�����ϡ��������β�������µ��õ�©���л������ã���ֹ������
��4���Ȼ�狀�������þ�������ֽⷴӦ���ɼ�ʽ�Ȼ�þ[Mg��OH��Cl]��ͬʱ���ɰ�����ˮ����Ӧ����ʽΪMg��OH��2+NH4Cl![]() MgOHCl+NH3��+H2O���ʴ�Ϊ��Mg��OH��2+NH4Cl
MgOHCl+NH3��+H2O���ʴ�Ϊ��Mg��OH��2+NH4Cl![]() MgOHCl+NH3��+H2O��
MgOHCl+NH3��+H2O��
��4����ʯ��������ˮ���Ӷ������������ʯ�ҺͰ�������Ӧ�����Լ�ʯ���ܸ��ﰱ�����ʴ�Ϊ����ʯ�ң�
��6���������ܶ�С�ڿ�����C�е�����Ӧ�̽��������ʴ�Ϊ��C�е�����Ӧ�̽�������
��7������MgCl2��Һ����ʱˮ��̶ȼӴ����ɵ��Ȼ���ӷ���ֱ�Ӽ��ȵò���MgCl26H2O���壬��Ҫ��HCl�����м����������ʴ�Ϊ����HCl�����м��ȡ�