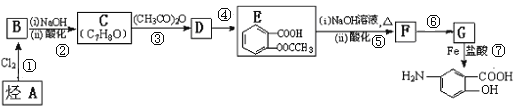
��Ŀ����
����Ŀ��A��B��C��D��E��FΪԪ�����ڱ�ǰ�����ڵ�Ԫ�أ�ԭ��������������AԪ�صĵ����ǿ�������Ҫ�ɷ֣�Bԭ�Ӻ���p�������1�ԳɶԵ��ӣ�DԪ�صļ۵������������������һ�룬C��Bͬ���壬A��Fͬ���壬D��Eͬ�塣�ش��������⣺
��1��A��B��C�ĵ�һ�������ɴ�С��˳��Ϊ_______________����Ԫ�ط��ű�ʾ����
��2��B��C�γɵĶ�Ԫ�������У����ڷǼ��Է��ӵ���________���ѧʽ�����÷�������ԭ�ӵ��ӻ��������Ϊ_____________��
��3��A��C�ֱ��γɵij����ĺ���������У�����ԭ�ӵļ۲���Ӷ���Ϊ4������______���ѧʽ����ͬ���������ƽ�������ε�����________________��
��4��Dn+��Br-��C����ۺ��������A�ļ��⻯�ﰴ1:1:1:5�γ�ij���������������Һ�еμ�AgNO3��Һ��������ɫ�������μ�BaCl2��Һ���������������е�����Ϊ___________��nֵΪ__________��Dn+�Ļ�̬�����Ų�ʽΪ____________��
��5������EB����Ľṹ��ͼ��ʾ���侧���߳�Ϊapm����ʽ��ʾEB������ܶ�Ϊ__________g��cm-3�����ؼ�������������٤��������ֵΪNA�����˹��Ʊ���EB�����г�����ȱ�ݣ�һ��E2+��ȱ����������E2+������E3+��ȡ�������������Գʵ����ԣ�����������E��B�ı�ֵȴ�����˱仯����֪ij��������Ʒ���E0.96B���þ�����E3+��E2+�����Ӹ���֮��Ϊ_____________��

���𰸡�
��1��N>O>S��
��2��SO3��sp2��
��3��H2SO3��H2SO4��HNO2��
��4��SO42-��NH3��3��1s22s22p63s23p63d6����[Ar]3d6��
��5��![]() ��1:11
��1:11
��������
���������A��B��C��D��E��FΪԪ�����ڱ�ǰ�����ڵ�Ԫ�أ�ԭ��������������AԪ�صĵ����ǿ�������Ҫ�ɷ֣�Bԭ�Ӻ���p�������1�ԳɶԵ��ӣ���BΪOԪ�أ�AΪNԪ�أ�DԪ�صļ۵������������������һ�룬DΪCoԪ�أ�C��Bͬ���壬CΪSԪ�أ�A��Fͬ���壬��FΪAsԪ�أ�D��Eͬ�壬��EΪNiԪ�ء�
��1���ǽ�����ǿ�ĵ�һ�����ܴ�N��2p���Ӱ���Ϊ�ȶ��ṹ����a��b��c�У���һ�������ɴ�С����ΪN��O��S�� �ʴ�Ϊ��N��O��S��
��2��B��C�γɵĶ�Ԫ��������SO2��SO3������SO2��SԪ�ز���sp2�ӻ���ΪV�ͽṹ�����ڼ��Է��ӣ�SO3��SԪ�ز���sp2�ӻ���Ϊƽ���������νṹ�����ڷǼ��Է��ӣ��ʴ�Ϊ��SO3��sp2��
��3��A��C�ֱ��γɵij����ĺ��������Ϊ����������������������ᣬ����������Nԭ�ӵļ۲���Ӷ���Ϊ3+![]() ����5-1-2��2��=3����������Nԭ�ӵļ۲���Ӷ���Ϊ2+
����5-1-2��2��=3����������Nԭ�ӵļ۲���Ӷ���Ϊ2+![]() ����5-1-2��=3��������Sԭ�ӵļ۲���Ӷ���Ϊ4+
����5-1-2��=3��������Sԭ�ӵļ۲���Ӷ���Ϊ4+![]() ����6-1��2-2��2��=4����������Sԭ�ӵļ۲���Ӷ���Ϊ3+
����6-1��2-2��2��=4����������Sԭ�ӵļ۲���Ӷ���Ϊ3+![]() ����6-1��2-2��=4������ԭ�ӵļ۲���Ӷ���Ϊ4����������������ᣬ�����ƽ�������ε����������ᣬ�ʴ�Ϊ��H2SO3��H2SO4��HNO2��
����6-1��2-2��=4������ԭ�ӵļ۲���Ӷ���Ϊ4����������������ᣬ�����ƽ�������ε����������ᣬ�ʴ�Ϊ��H2SO3��H2SO4��HNO2��
��4�� Con+��Br-�������������1:1:1:5�γ�ij���������������Һ�еμ�AgNO3��Һ��������ɫ������˵������������磬�μ�BaCl2��Һ������˵��������ڃȽ磬������е�����ΪSO42-��NH3�����ݵ���غ㣬n=1+2=3��Co3+�Ļ�̬�����Ų�ʽΪ1s22s22p63s23p63d6���ʴ�Ϊ��SO42-��NH3��3��1s22s22p63s23p63d6��
��5��NiO�ľ���Ϊ�������Ľṹ�������к�OΪ8��![]() +6��
+6��![]() =4����NiΪ1+12��
=4����NiΪ1+12��![]() =4�������߳�Ϊa pm�����Ϊ��a��10-10cm��3���þ������ܶ�Ϊ
=4�������߳�Ϊa pm�����Ϊ��a��10-10cm��3���þ������ܶ�Ϊ![]() gcm-3=
gcm-3=![]() gcm-3����1mol Ni0.96O�к�Ni3+xmol��Ni2+Ϊ��0.96-x��mol�����ݾ����Գʵ����ԣ���֪ 3x+2����0.96-x��=2��1�����x=0.08mol��Ni2+Ϊ��0.96-x��mol=0.88mol����������֮��ΪNi3+��Ni2+=0.08��0.88=1��11���ʴ�Ϊ��
gcm-3����1mol Ni0.96O�к�Ni3+xmol��Ni2+Ϊ��0.96-x��mol�����ݾ����Գʵ����ԣ���֪ 3x+2����0.96-x��=2��1�����x=0.08mol��Ni2+Ϊ��0.96-x��mol=0.88mol����������֮��ΪNi3+��Ni2+=0.08��0.88=1��11���ʴ�Ϊ��![]() ��1��11��
��1��11��

 ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д� ����5��2���ϵ�д�
����5��2���ϵ�д�