��Ŀ����
17��ˮ��һ����Ҫ����Ȼ��Դ���������������治��ȱ�ٵ����ʣ�ˮ������ֱ��Ӱ�����彡������ش��������⣺��1����������ˮ�Ĵ���ʱ��������еķ����Dzⶨˮ�ĵ絼�ʻ�����ʣ�
��2��ˮ�ľ�����������������ˮ�ľ������û��������������ȣ�ʹʹˮ�н��弰�����������������ˮ�������dz�ȥˮ�и����Ӻ�þ���ӣ�
��3��ͨ��ʩ��һ��ѹ��ʹˮ����ͨ����Ĥ��������ӻ����ӽ������Ӷ���ô���ˮ�ķ�����Ϊ��������������������ˮʱ��ʹ����ͨ����Ĥ���ƶ����ǵ��Ʋ��糡����
��4��Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mgCaO����֮�൱�����ʣ���7.1mgMgO������ij��Ȼˮ��c��Ca2+��=1.0��10-3mol•L-1��c��Mg2+��=6��10-4mol•L-1���ˮ��Ӳ��Ϊ8.98�㣮
��5������RH�������ӽ�����֬��ROH�������ӽ�����֬������������ȻӲˮ��Ӧ��ʹӲˮ��ͨ��RH���RH����ROH���������ӽ�����֬��ԭ������ͨ�������ӽ�����֬����Mg��OH��2�ȳ���Ӱ�콻��Ч����
���� ��1����Ϊˮ�ĵ���̶ȼ�С�����Դ�ˮ�Ǽ���������ģ�
��2������ˮ�����ɽ�����������ԣ������ǽ������Ӻ�þ���ӵ�Ũ�ȣ�
��3��ʩ��һ��ѹ��ʹˮ����ͨ����Ĥ��������ӻ����ӽ���������ԭ����ѹǿ�йأ�������������ˮʱ������ԭ�����ѹ�йأ�
��4��Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mgCaO����֮�൱�����ʣ���7.1mgMgO������ˮ�е�Ca2+��Mg2+���������CaO���������㣻
��5��RH�������ӽ�����֬��ROH�������ӽ�����֬������������ȻӲˮʱ��RH�������ӽ�����֬�ɽ���Ӳˮ�е�Ca2+��Mg2+���ӣ�����������Mg��OH��2�ȳ�����Ӱ����֬����Ч����
��� �⣺��1����Ϊˮ�ĵ���̶ȼ�С�����Դ�ˮ�Ǽ���������ģ����Ҫ��������ˮ�Ĵ���ʱ��������еķ����Dzⶨˮ�ĵ絼�ʻ�����ʣ��ʴ�Ϊ���絼�ʣ�������ʣ���
��2��ˮ�ľ�����������������ˮ�ľ������û��������������ȣ�ʹˮ�н��弰�����������������ˮ�������dz�ȥˮ�и����Ӻ�þ���ӣ�
�ʴ�Ϊ��ʹˮ�н��弰�����������������ȥˮ�и����Ӻ�þ���ӣ�
��3��ͨ��ʩ��һ��ѹ��ʹˮ����ͨ����Ĥ��������ӻ����ӽ������Ӷ���ô���ˮ�ķ�����Ϊ������������ӵ糡�������£�ˮ��Һ�����������ӻ�ֱ��������ƶ���������м����һ�ֽ���Ĥ���Ϳ��ܴﵽ����Ũ����Ŀ�ģ��������������ˮʱ��ʹ����ͨ����Ĥ���ƶ����ǵ��Ʋ��糡����
�ʴ�Ϊ�������������Ʋ��糡������
��4��ij��Ȼˮ��c��Ca2+��=1.0��10-3mol•L-1��c��Mg2+��=6��10-4mol•L-1��Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mgCaO����֮�൱�����ʣ���7.1mgMgO����1Lˮ�и��������ʵ���=1.0��10-3mol���൱��CaO����=1.0��10-3mol��56g/mol=56mg��1Lˮ��þ�������ʵ���=6��10-4mol���൱������þ����6��10-4mol��40g/mol=24mg������ˮ��Ӳ��=$\frac{56mg}{10mg}$+$\frac{24mg}{7.1mg}$��8.98��ʴ�Ϊ��8.98�㣻
��5�����ˮ��Ӳ�����ɸƺ�þ�������λ��Ȼ��������ģ�����Ӳ�Ƚ�������Ӳ�ȣ���������Ӳ�ȵ�ˮ���Բ������ӽ������������������ӽ���������������ˮ�е�Ca2+��Mg2+�������ӽ������ã�ʹˮ�õ����������Ӳˮ��ͨ��ROH�������ӽ�����֬ʱ���ܲ���Mg��OH��2�ȳ�����Ӱ�콻��Ч����������ͨ��RH�������ӽ�����֬��
�ʴ�Ϊ��RH����ͨ�������ӽ�����֬��������Mg��OH��2�ȳ�����Ӱ����֬����Ч����
���� ���⿼����ۺϣ��漰����ˮ�⡢ˮ�ľ��������������ᴿ�ȣ�Ϊ��Ƶ���㣬���շ�Ӧԭ��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

| A�� | 40K��40Caԭ���е�������������������� | |
| B�� | ijԪ��ԭ�������ֻ���������ӣ���һ���ǽ���Ԫ�� | |
| C�� | �κ�ԭ�ӻ����ӵ�����ж��������� | |
| D�� | ͬλ�صIJ�ͬ������������ѧ������ȫ��ͬ |
| A�� | KSCN | B�� | BaCl2 | C�� | NaOH | D�� | HCl |
| A�� | CCl2�TCH2 | B�� | CH3-CH�TCH2 | C�� | CH3-CH�TCH-CH3 | D�� | CH2�TCH-CH2-CH3 |
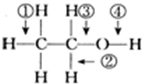
| A�� | �� | B�� | �� | C�� | �� | D�� | �� |
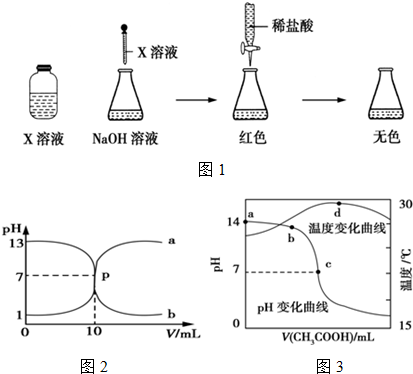
 ����A��D��Ԫ�ؿ������X��Y���ֻ����X��һ�������¿��Էֽ��Y��X��������ѧ�����ͼ��Լ����Ǽ��Լ�����X�м�������KMnO4��Һ��д������ǡ�÷�Ӧ�����ӷ���ʽ5H2O2+2MnO4-+6H+=5O2��+2Mn2++8H2O�� ������E2 D2�뻯����E2H��ˮ��Һ�����ʵ�����1��1��Ӧ���ɵ���H�Ļ�ѧ����ʽΪNa2O2+2H2O+Na2S=S��+4NaOH��
����A��D��Ԫ�ؿ������X��Y���ֻ����X��һ�������¿��Էֽ��Y��X��������ѧ�����ͼ��Լ����Ǽ��Լ�����X�м�������KMnO4��Һ��д������ǡ�÷�Ӧ�����ӷ���ʽ5H2O2+2MnO4-+6H+=5O2��+2Mn2++8H2O�� ������E2 D2�뻯����E2H��ˮ��Һ�����ʵ�����1��1��Ӧ���ɵ���H�Ļ�ѧ����ʽΪNa2O2+2H2O+Na2S=S��+4NaOH��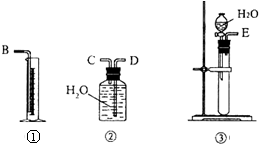 ���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ��ͼ�е�װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȣ�
���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ��ͼ�е�װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȣ�