��Ŀ����
ij��ѧʵ������Ҫ0.2mol/LNaOH��Һ500mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����______������ţ�������������Һ�����õ��IJ���������______�����������ƣ���
��2������ƿ��������Һ����Ҫ����������ƿ�ϱ������������е�______����д��ţ���
���¶Ȣ�Ũ�Ȣ�������ѹǿ����ʽ���ʽ�̶���
��3������ʱ������ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�______��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ50mL�����ò���������������ʹ�����ܽ⣬��ȴ������
C��������ƿ�ǽ���ҡ��
D�����ܽ������������Һ�ز�����ע������ƿ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2��3cm��
��4�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ��______mL������������һλС������
��5�����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ���������ǣ�______��
��6���������Ƶ�ϡH2SO4���вⶨ������ʵ��Ũ��С��0.5mol/L���������������Щ��������������Ũ��ƫС����д��ĸ��______��
A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B������ƿδ���T����������Һ
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж���
D��������ƿת��ʱ��������Һ�彦��
E��������ƿ�ж���ʱ��������ƿ�̶���
F���ձ�δ����ϴ��
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�
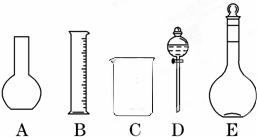
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����______������ţ�������������Һ�����õ��IJ���������______�����������ƣ���
��2������ƿ��������Һ����Ҫ����������ƿ�ϱ������������е�______����д��ţ���
���¶Ȣ�Ũ�Ȣ�������ѹǿ����ʽ���ʽ�̶���
��3������ʱ������ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�______��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ50mL�����ò���������������ʹ�����ܽ⣬��ȴ������
C��������ƿ�ǽ���ҡ��
D�����ܽ������������Һ�ز�����ע������ƿ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2��3cm��
��4�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ��______mL������������һλС������
��5�����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ���������ǣ�______��
��6���������Ƶ�ϡH2SO4���вⶨ������ʵ��Ũ��С��0.5mol/L���������������Щ��������������Ũ��ƫС����д��ĸ��______��
A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B������ƿδ���T����������Һ
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж���
D��������ƿת��ʱ��������Һ�彦��
E��������ƿ�ж���ʱ��������ƿ�̶���
F���ձ�δ����ϴ��
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�

��1��AΪƽ����ƿ��DΪ��Һ©������������Һ�����в���ʹ�õ���ƿ�ͷ�Һ©��������һ��Ũ�ȵ���Һ��ȱ�ٲ������ͽ�ͷ�ιܣ�
�ʴ�Ϊ��AD������������ͷ�ιܣ�
��2������ƿΪ��������������ƿ�ϱ����¶ȡ������Ϳ̶��ߣ����Ԣ٢ۢ���ȷ��
�ʴ�Ϊ���٢ۢޣ�
��3������һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ��BDAFEC��
�ʴ�Ϊ��BDAFEC��
��4����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ�����Ũ��Ϊ��
mol/L=18.4mol/L��ʵ����û��450mL������ƿ��ʵ�������Ƶ���500��mL 0.5mol/L�����ᣬ��ҪŨ��������Ϊ��
��0.0136L=13.6mL��
�ʴ�Ϊ��13.6��
��5��ϡ��Ũ����ʱ���뽫Ũ�������ˮ�У���ȷ�IJ�������Ϊ����Ũ���������ձ��ڣ�����������������ˮ�У��������ò��������裬
�ʴ�Ϊ����Ũ���������ձ��ڣ�����������������ˮ�У��������ò��������裻
��6��A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶ȣ�������ȡ��Ũ�������ƫ�����Ƶ���ҺŨ��ƫ�ߣ���A����
B������ƿδ���T����������Һ�������ʵ����ʵ�������Һ�������û��Ӱ�죬���Բ�Ӱ�����ƽ������B����
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж��ݣ��ȵ���Һ���ƫ����ȴ�������С�����Ƶ���Һ���ƫС������c=
����ҺŨ��ƫ�ߣ���C����
D��������ƿת��ʱ��������Һ�彦�������Ƶ���Һ�����ʵ����ʵ���ƫС������c=
�����Ƶ���ҺŨ��ƫ�ͣ���D��ȷ��
E��������ƿ�ж���ʱ��������ƿ�̶��ߣ����¼��������ˮ�����������ƿ�̶��ߣ����Ƶ���Һ���ƫС������c=
����Һ��Ũ��ƫ�ߣ���E����
F���ձ�δ����ϴ�ӣ��������Ƶ���Һ�����ʵ����ʵ���ƫС������c=
�����Ƶ���ҺŨ��ƫ�ͣ���F��ȷ��
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ����Ƶ���Һ���ƫ����c=
����Һ��Ũ��ƫ�ͣ���G��ȷ��
�ʴ�Ϊ��DFG��
�ʴ�Ϊ��AD������������ͷ�ιܣ�
��2������ƿΪ��������������ƿ�ϱ����¶ȡ������Ϳ̶��ߣ����Ԣ٢ۢ���ȷ��
�ʴ�Ϊ���٢ۢޣ�
��3������һ�����ʵ���Ũ�ȵ���Һ�IJ���Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ��BDAFEC��
�ʴ�Ϊ��BDAFEC��
��4����������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ�����Ũ��Ϊ��
| 1000��1.84��98% |
| 98 |
| 0.5mol/L��0.5L |
| 18.4mol/L |
�ʴ�Ϊ��13.6��
��5��ϡ��Ũ����ʱ���뽫Ũ�������ˮ�У���ȷ�IJ�������Ϊ����Ũ���������ձ��ڣ�����������������ˮ�У��������ò��������裬
�ʴ�Ϊ����Ũ���������ձ��ڣ�����������������ˮ�У��������ò��������裻
��6��A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶ȣ�������ȡ��Ũ�������ƫ�����Ƶ���ҺŨ��ƫ�ߣ���A����
B������ƿδ���T����������Һ�������ʵ����ʵ�������Һ�������û��Ӱ�죬���Բ�Ӱ�����ƽ������B����
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж��ݣ��ȵ���Һ���ƫ����ȴ�������С�����Ƶ���Һ���ƫС������c=
| n |
| V |
D��������ƿת��ʱ��������Һ�彦�������Ƶ���Һ�����ʵ����ʵ���ƫС������c=
| n |
| V |
E��������ƿ�ж���ʱ��������ƿ�̶��ߣ����¼��������ˮ�����������ƿ�̶��ߣ����Ƶ���Һ���ƫС������c=
| n |
| V |
F���ձ�δ����ϴ�ӣ��������Ƶ���Һ�����ʵ����ʵ���ƫС������c=
| n |
| V |
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ����Ƶ���Һ���ƫ����c=
| n |
| V |
�ʴ�Ϊ��DFG��

��ϰ��ϵ�д�
�����Ŀ