��Ŀ����
ʵ����Ҫ����100mL��10mol?L-1��NaCl��Һ���Իش����и��⣺
��1�������㣬Ӧ����������ƽ��ȡNaCl����______g��
��2��������Һʱһ��ɷ�Ϊ���¼������裺�ٳ����ڼ���۶��ݢ���Һ��ϴ�Ӣ��ܽ�����ȷ�IJ���˳��Ϊ______��
��3����ʵ�������õ��������������÷ֱ��ǣ�
���ܽ�ʱ������������______����Һʱ������������______
��4������ƿ�����������5���еĢ��¶Ȣ�Ũ�Ȣ�������ѹǿ�ݿ̶���______
A���٢ۢ�B���ۢݢ�C���٢ڢ�D���ڢܢ�
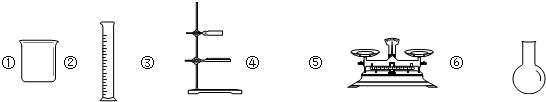
��5������NaCl����������Һ�����������У�����Ҫ�õ�����______��������ţ�
A��������B.100mL����ƿC���ձ�D����ͷ�ι�
E��ҩ��F��������ƽG��������
��6�����д�������ᵼ��������ҺŨ��ƫ�͵���______���������ţ���
A������ʱ��������ƿ�̶���
B������ƿ��ԭ����������ˮ
C�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�
D�����ƺ���Һ������ƿδ���ã�����һЩ��Һ��
��1�������㣬Ӧ����������ƽ��ȡNaCl����______g��
��2��������Һʱһ��ɷ�Ϊ���¼������裺�ٳ����ڼ���۶��ݢ���Һ��ϴ�Ӣ��ܽ�����ȷ�IJ���˳��Ϊ______��
��3����ʵ�������õ��������������÷ֱ��ǣ�
���ܽ�ʱ������������______����Һʱ������������______
��4������ƿ�����������5���еĢ��¶Ȣ�Ũ�Ȣ�������ѹǿ�ݿ̶���______
A���٢ۢ�B���ۢݢ�C���٢ڢ�D���ڢܢ�
��5������NaCl����������Һ�����������У�����Ҫ�õ�����______��������ţ�
A��������B.100mL����ƿC���ձ�D����ͷ�ι�
E��ҩ��F��������ƽG��������
��6�����д�������ᵼ��������ҺŨ��ƫ�͵���______���������ţ���
A������ʱ��������ƿ�̶���
B������ƿ��ԭ����������ˮ
C�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�
D�����ƺ���Һ������ƿδ���ã�����һЩ��Һ��
��1��NaCl�����ʵ���n=cV=0.1L��10mol?L-1=1mol��NaCl������Ϊ1mol��58.5g/mol=58.5g��
�ʴ�Ϊ��58.5��
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�����Һ��Һ����̶���ˮƽ���У��Ǻ�ƿ���������ߵ�ҡ�ȣ�����ȷ����˳��Ϊ�ڢ٢ޢܢݢۣ�
�ʴ�Ϊ���ڢ٢ޢܢݢۣ�
��3�����ܽ�ʱ�������������ǽ�������ܽ⣻����Һʱ��������������������
�ʴ�Ϊ�����������Һ��������
��4������ƿ�ϱ��У��¶ȡ��������̶��ߣ�
��ѡA��
��5���ɣ�2���в������ƿ�֪������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ��ʲ���Ҫ��������
�ʴ�Ϊ��A��
��6��A������ʱ��������ƿ�̶��ߣ���Һ��Һ�泬���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���A���ϣ�
B����Һ�������ˮ���ݣ�����ƿ��ԭ����������ˮ����������ҺŨ����Ӱ�죬��B�����ϣ�
C�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ٲ��伸��ˮ���̶ȴ�������������Һ�����ƫ��������ҺŨ��ƫ�ͣ���C���ϣ�
D����Һ�Ǿ��ȵģ����ƺ���Һ������ƿδ���ã�����һЩ��Һ��ʣ����Һ��Ũ����ԭ��ҺŨ����ͬ����D�����ϣ�
��ѡAC��
�ʴ�Ϊ��58.5��
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�����Һ��Һ����̶���ˮƽ���У��Ǻ�ƿ���������ߵ�ҡ�ȣ�����ȷ����˳��Ϊ�ڢ٢ޢܢݢۣ�
�ʴ�Ϊ���ڢ٢ޢܢݢۣ�
��3�����ܽ�ʱ�������������ǽ�������ܽ⣻����Һʱ��������������������
�ʴ�Ϊ�����������Һ��������
��4������ƿ�ϱ��У��¶ȡ��������̶��ߣ�
��ѡA��
��5���ɣ�2���в������ƿ�֪������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ��ʲ���Ҫ��������
�ʴ�Ϊ��A��
��6��A������ʱ��������ƿ�̶��ߣ���Һ��Һ�泬���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���A���ϣ�
B����Һ�������ˮ���ݣ�����ƿ��ԭ����������ˮ����������ҺŨ����Ӱ�죬��B�����ϣ�
C�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ٲ��伸��ˮ���̶ȴ�������������Һ�����ƫ��������ҺŨ��ƫ�ͣ���C���ϣ�
D����Һ�Ǿ��ȵģ����ƺ���Һ������ƿδ���ã�����һЩ��Һ��ʣ����Һ��Ũ����ԭ��ҺŨ����ͬ����D�����ϣ�
��ѡAC��

��ϰ��ϵ�д�
�����Ŀ