��Ŀ����
ijͬѧ��ѧϰ�������˽���ǵ���Ҫ�ɷ���̼��ƣ����ǣ���ͬѧ�����Dz������еõ����еĺ�������һ���������һ���Ļ�����Լ�Ƕ��٣������һ���Ļ��������еĺ�������ʲô�����й��أ����Ǹ�ͬѧ���Դ�Ϊ����������ͬѧһ��չ��һ���о���ѧϰ�����о���ѧϰ�У���ͬѧ�ķֹ�����Ʋ��������к������ķ���������������Ƶ�һ������������Ϊ����ȫ���������ش�������⣮
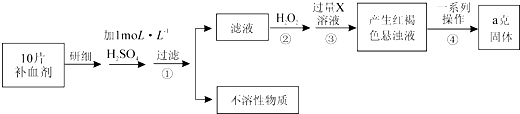
��һ�����ռ����ǣ���ϴ�Ӹɾ����ã�
�ڶ�������������������m1����
���������ܽ⵰�ǣ������Ƿ���ʢ��______���ձ��У����裬ʹ���ַ�Ӧ�����˳�ȥ���������ʣ�������Һ���ã������ܽ�����з��������ӷ���ʽΪ______��
���IJ�������Ca2+������Һ�м��������______��Һ��Ҫ��������ĸ���Һ��ԭ����______��
���岽�����ˣ�ϴ�ӳ�����ϴ�ӳ�����ԭ��ϴ�ӵķ�����______��
����������������������m2���������к�����������Ԫ�ص������������ı���ʽΪCa%=______��
��һ�����ռ����ǣ���ϴ�Ӹɾ����ã�
�ڶ�������������������m1����
���������ܽ⵰�ǣ������Ƿ���ʢ��______���ձ��У����裬ʹ���ַ�Ӧ�����˳�ȥ���������ʣ�������Һ���ã������ܽ�����з��������ӷ���ʽΪ______��
���IJ�������Ca2+������Һ�м��������______��Һ��Ҫ��������ĸ���Һ��ԭ����______��
���岽�����ˣ�ϴ�ӳ�����ϴ�ӳ�����ԭ��ϴ�ӵķ�����______��
����������������������m2���������к�����������Ԫ�ص������������ı���ʽΪCa%=______��
�������������к���̼��ƣ����������ᣬ����CaCO3+2H+=Ca2++CO2��+H2O��
�ʴ�Ϊ�����CaCO3+2H+=Ca2++CO2��+H2O��
���IJ���Ϊʹ������ȫ��������Ӧ���������Na2CO3������̼��Ƶ�������ȷ�������к�������
�ʴ�Ϊ��Na2CO3��ʹCa2+��ȫ������
���岽����������������ã����������������������ӣ�������ˮ��ϴ��Ӧ��Сʵ����
�ʴ�Ϊ����Ϊ���������������������ӣ�������ˮ��ϴ��
��������������������Ϊm2����m��Ca��=
g��
�����к�����Ϊ
��100%=
��100%��
�ʴ�Ϊ��
��100%��
�ʴ�Ϊ�����CaCO3+2H+=Ca2++CO2��+H2O��
���IJ���Ϊʹ������ȫ��������Ӧ���������Na2CO3������̼��Ƶ�������ȷ�������к�������
�ʴ�Ϊ��Na2CO3��ʹCa2+��ȫ������
���岽����������������ã����������������������ӣ�������ˮ��ϴ��Ӧ��Сʵ����
�ʴ�Ϊ����Ϊ���������������������ӣ�������ˮ��ϴ��
��������������������Ϊm2����m��Ca��=
| 40m2 |
| 100 |
�����к�����Ϊ
| ||
| m1 |
| 40m2 |
| 100m1 |
�ʴ�Ϊ��
| 40m2 |
| 100m1 |

��ϰ��ϵ�д�
�����Ŀ