��Ŀ����
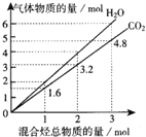
����Ŀ����1����֪0.05molij���������Ŀ�������ȫȼ����������ͨ�������ij���ʯ��ˮ���õ���ɫ����30.0g���������ļ�ʯ������ȼ�ղ������18.6g���������ʵ��ʽΪ___________��;����ʽΪ_______;
����������������̼ԭ�Ӷ���һ��ƽ���ϣ���ṹ��ʽΪ________________________
��2��ij�л��ᆳ���ϣ��������������֪����Է�������Ϊ136������ʽC8H8O2��������ֻ��һ�������ұ�����ֻ��һ��ȡ��������˴Ź���������ͼ����A���ܵĽṹ��ʽ_____________________

��3��ij���ĺ���������������С��150�����к���Ԫ�ص���������Ϊ50%����������е���ԭ�Ӹ�������Ϊ____________������ԭ�������ĸ��л���ķ���ʽΪ____________________________
���𰸡� CH2 C6H12 ![]()
![]() ��
��![]() 1��4 C5H4O4
1��4 C5H4O4
����������1����ɫ������̼��ƣ����ʵ�����30g��100g/mol��0.3mol����CO2�����ʵ�����0.3mol��������0.3mol��44g/mol��13.2g����ʯ������ˮ��CO2����ˮ��������18.6g��13.2g��5.4g�����ʵ�����5.4g��18g/mol��0.3mol������C��H��ԭ�Ӹ���֮����1��2�����ʵ��ʽ��CH2��������̼ԭ�Ӹ�����0.3mol��0.05mol��6�����Է���ʽΪC6H12������������������̼ԭ�Ӷ���һ��ƽ���ϣ���ṹ��ʽΪ![]() ����2������ͼʾ��֪�����к���4����ԭ�ӣ����ں�һ�������ұ�����ֻ��һ��ȡ���������Ը�ȡ����Ӧ���ǣ�COCH3���ǣ�OOCCH3����A���ܵĽṹ��ʽΪ
����2������ͼʾ��֪�����к���4����ԭ�ӣ����ں�һ�������ұ�����ֻ��һ��ȡ���������Ը�ȡ����Ӧ���ǣ�COCH3���ǣ�OOCCH3����A���ܵĽṹ��ʽΪ![]() ��
��![]() ����3���������1����ԭ�ӣ�����Է���������16��0.5��32����Ϊ�״����������2����ԭ�ӣ�����Է���������32��0.5��64���������2��̼ԭ�ӣ�����ԭ����8��������̼ԭ�ӵ��ļ����ۿ�֪���л��ﲻ���ڣ��������3����ԭ�ӣ�����Է���������48��0.5��96���������3��̼ԭ�ӣ�����ԭ����12��������̼ԭ�ӵ��ļ����ۿ�֪���л��ﲻ���ڣ��������4����ԭ�ӣ�����Է���������64��0.5��128����ʱ̼ԭ�Ӹ���Ӧ����5������ԭ�Ӹ�����4�������Է���ʽΪC5H4O4���������5����ԭ�ӣ�����Է�����������150����������
����3���������1����ԭ�ӣ�����Է���������16��0.5��32����Ϊ�״����������2����ԭ�ӣ�����Է���������32��0.5��64���������2��̼ԭ�ӣ�����ԭ����8��������̼ԭ�ӵ��ļ����ۿ�֪���л��ﲻ���ڣ��������3����ԭ�ӣ�����Է���������48��0.5��96���������3��̼ԭ�ӣ�����ԭ����12��������̼ԭ�ӵ��ļ����ۿ�֪���л��ﲻ���ڣ��������4����ԭ�ӣ�����Է���������64��0.5��128����ʱ̼ԭ�Ӹ���Ӧ����5������ԭ�Ӹ�����4�������Է���ʽΪC5H4O4���������5����ԭ�ӣ�����Է�����������150����������