��Ŀ����
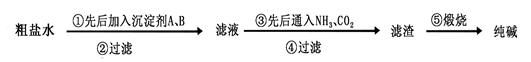
�ִ�п��Ʒ�ӹ���ҵ���յķ���������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�����ȡ����п���������£�
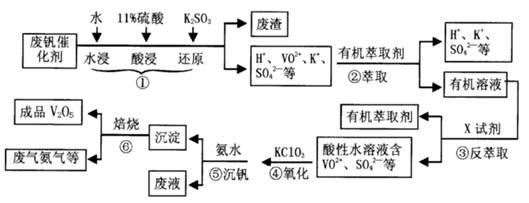
�й�����������ȫ������pH���±���
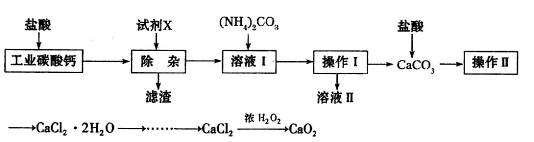
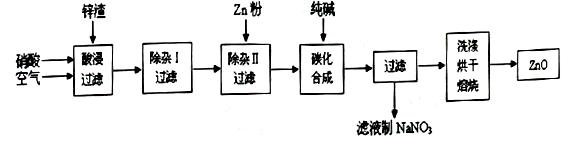
��l������������У�Ҫ���пԪ�صĽ����ʣ����Բ�ȡ ��ʩ��
��2�����������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ��������� ��
��3���ڡ�����I�������У�����Һ����pH=4��Ŀ���� ���ڡ�����II������Һ��pHԼΪ6����˲�����ʱ�����к��� ��
��4���ڡ�̼���ϳɡ��У����ɵIJ���֮һΪ��ʽ̼��п[Zn2��OH��2CO3]��ͬʱ�ų�CO2����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5������Һ����ȡNaNO3����IJ�������Ϊ ��
��6����ʵ�������ϴ�ӹ��˳��ļ�ʽ̼��п�� ��

�й�����������ȫ������pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 | 8.0 |
��l������������У�Ҫ���пԪ�صĽ����ʣ����Բ�ȡ ��ʩ��
��2�����������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ��������� ��
��3���ڡ�����I�������У�����Һ����pH=4��Ŀ���� ���ڡ�����II������Һ��pHԼΪ6����˲�����ʱ�����к��� ��
��4���ڡ�̼���ϳɡ��У����ɵIJ���֮һΪ��ʽ̼��п[Zn2��OH��2CO3]��ͬʱ�ų�CO2����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5������Һ����ȡNaNO3����IJ�������Ϊ ��
��6����ʵ�������ϴ�ӹ��˳��ļ�ʽ̼��п�� ��
��16�֣�
��1�����ȡ����裨2�֣�
��2���ձ�����������©�� ��3�֣�
��3��ʹFe3+ת��ΪFe(OH)3������ȥ��2�֣�
Al(OH)3��Cu��Zn ��2�֣� ������Zn���۷֣�
��4��2Na2CO3+2Zn(NO3)2+H2O=4NaNO3+Zn2(OH)2CO3+CO2����2�֣�
��5������Ũ������ȴ�ᾧ�����ˣ�3�֣�
��6����©���м�����������ˮ����û������������Ȼ�˳����������Σ�2�֣�
��1�����ȡ����裨2�֣�
��2���ձ�����������©�� ��3�֣�
��3��ʹFe3+ת��ΪFe(OH)3������ȥ��2�֣�
Al(OH)3��Cu��Zn ��2�֣� ������Zn���۷֣�
��4��2Na2CO3+2Zn(NO3)2+H2O=4NaNO3+Zn2(OH)2CO3+CO2����2�֣�
��5������Ũ������ȴ�ᾧ�����ˣ�3�֣�
��6����©���м�����������ˮ����û������������Ȼ�˳����������Σ�2�֣�
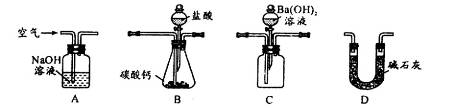
�����������1������Ӧ��Ũ�ȡ������¶ȡ�����ѹǿ��������μӵķ�Ӧ�����������������ʹ�ú��ʵĴ�������ĥ�����衢�ȴ�ʩ�����Ǽӿ췴Ӧ���ʡ���߽����ʵij��ô�ʩ����2�����˷����Һ�������õIJ����������ձ�����ͨ©��������������3���������ǿ�����Ժ�ǿ���ԣ����������¿����е�����Ҳ�ܽ�������������Ϊ�����ӣ�����Һ�к���Zn2+��Fe3+��Cu2+��Al3+��H+��NO3������������Ϣ��֪������ҺpH����4ʱ����������ȫ��Ϊ�����������������Գ�ȥ��Һ�е������ӣ�����ҺpH����6ʱ����������ȫ��Ϊ�����������������Գ�ȥ��Һ�е������ӣ�����п��ͭ���ã�����������п���������������ӣ������ܽ�ͭ������ȫ��ԭΪ����ͭ���ȳ�ȥ���ʣ���û�����������ʣ������II����������Ҫ�ɷ�Ϊ����������ͭ��п����4�������⣬�����̼����������п��Һ��Ӧ�����ɼ�ʽ̼�ᡢ������̼�����������غ�ԭ���ɵøø��ֽⷴӦ����ʽ��2Na2CO3+2Zn(NO3)2+H2O=4NaNO3+Zn2(OH)2CO3+CO2������5���������ǿ������Σ��ܽ�����¶��½������Լ�С�������������ƶϣ�����Һ����Ũ������ȴ�ᾧ�����˵õ������ƾ��壻��6�����ݹ���֮��ϴ�ӳ�����һ��ԭ����ϴ�Ӽ�ʽ̼��пʱ������©���м�����������ˮ����û��������������Ȼ�˳����������μ��ɡ�

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
 2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��
2RAn���л��㣩 + nH2SO4 (ˮ��)Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�� ��