��Ŀ����
13�����ĺϳ�����Ҫ��һ�����������֪�ϳɰ��й������仯��ͼ����ͼ1��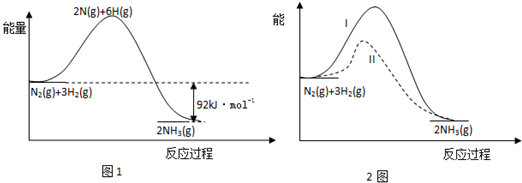
��1����Ӧ N2��g��+3H2��g��?2NH3��g����H=-92KJ/mol��
��2����ѧ��Ӧ����Ϊ�ɽ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ����1mol��ѧ��ʱ�ͷţ������գ��������������ṩ���»�ѧ���ļ��ܣ�kJ•mol-1����H-H��436��N��N��946����N-H�ļ�����
391kJ•mol-1��
��3����һ�������°�ͼ2ʵ��I���У��ı�ij������ͼ2����II���У�������������Ǽ��������
��4����һ���ܱ������м���1molN2��3mol H2��һ�������³�ַ�Ӧ������ЧӦʼ��С��92kJ����˵��ԭ����淴Ӧ������ȫת����
��5����һ���ܱ������м���amolN2��bmol H2���ﵽƽ��ʱn��N2����n��H2��=1��3����a��b=1��3��
��6����һ��5L�ܱ������м���8molN2��22mol H2��2min��v��N2��=0.2mol•L-1•min-1��5min��ﻯѧƽ�⣬ά���¶Ȳ���ƽ��ʱѹǿ�Ƿ�Ӧǰ��11/15.2min��ƽ��ʱn��NH3���ֱ���4mol��8mol����д���˽���ļ�����̣�
���� ��1����ͼ���֪����Ӧ�����������������Ӧ���ȣ���H=������������-��Ӧ�������ͣ�
��2���ݡ�H=��Ӧ����ܺ�-��������ܺ����㣻
��3����Ӧ�Ļ�ܽ����ˣ������ܹ����ͷ�Ӧ�Ļ�ܣ�
��4���ʱ����������뷴Ӧ����������淴Ӧ������ȫת�������ȱ��ʱ�С��
��5���ϳɰ���Ӧ�е��������������ʵ���֮��Ϊ1��3��ƽ��ʱΪ1��3��˵������ķ�Ӧ��֮��һ��Ϊ1��3��
��6����һ��5L�ܱ������м���8molN2��22mol H2��2min��v��N2��=0.2mol•L-1•min-1������2min�����ĵ���Ϊ0.2��5��2=2mol������N2+3H2?2NH3�������ɰ���Ϊ4mol����2minn��NH3����4mol��
5min��ﻯѧƽ�⣬�����
N2��g��+3H2��g��?2NH3��g���跴Ӧת��xmol����������
��ʼ 8 22 0
ת�� x 3x 2x
ƽ��8-x 22-3x 2x
����ƽ��ʱѹǿ֮�ȵ������ʵ���֮���з��̼��㣮
��� �⣺��1����ͼ���֪����H=������������-��Ӧ��������=-92KJ/mol���ʴ�Ϊ��-92KJ/mol��
��2����H=��Ӧ����ܺ�-��������ܺͣ�-92KJ/mol=3��436KJ/mol+946KJ/mol-6��Q��N-H��������Q��N-H��=391KJ/mol���ʴ�Ϊ��391��
��3��ʵ��I������II�Ļ�ܸߣ�������ͬ��˵��ʹ���˴�������Ϊ������ͨ�����ͷ�Ӧ�Ļ�ܼӿ췴Ӧ���ʵģ��ʴ�Ϊ�����������
��4�����淴Ӧ�ķ�Ӧ�ﲻ����ȫת�������Ⱦ��٣��ʴ�Ϊ�����淴Ӧ������ȫת����
��5���ϳɰ���Ӧ�е��������������ʵ���֮��Ϊ1��3��ƽ��ʱΪ1��3��˵������ķ�Ӧ��֮��һ��Ϊ1��3������a��b=1��3���ʴ�Ϊ��1��3��
��6����һ��5L�ܱ������м���8molN2��22mol H2��2min��v��N2��=0.2mol•L-1•min-1������2min�����ĵ���Ϊ0.2��5��2=2mol������N2+3H2?2NH3�������ɰ���Ϊ4mol����2minn��NH3����4mol��
5min��ﻯѧƽ�⣬�����
N2��g��+3H2��g��?2NH3��g���跴Ӧת��xmol����������
��ʼ 8 22 0
ת�� x 3x 2x
ƽ��8-x 22-3x 2x
��Ϊƽ��ʱѹǿ�Ƿ�Ӧǰ��11/15������$\frac{8-x+22-3x+2x}{8+22}$=$\frac{11}{15}$�����x=4mol
����ƽ��ʱn��NH3��Ϊ4��2=8mol���ʴ�Ϊ��4mol��8mol��
���� ���⿼�����ʱ���������㷽���������Է�Ӧ��Ӱ�졢���淴Ӧ�ķ�Ӧ�ȡ��������ȵ��ڻ�ѧ������֮�ȣ���Ŀ�ѶȲ���

| A�� | 21��5 | B�� | 4��1 | C�� | 3��1 | D�� | 11��3 |
��A��s��?B��g��+C��g����
��2C��g��?D��g��+E��g����
�ﵽƽ��ʱ��c��D��=0.5mol•L-1��c��C��=4mol•L-1������¶��·�Ӧ�ٵ�ƽ�ⳣ��Ϊ��������
| A�� | 25 | B�� | 20 | C�� | 16 | D�� | 9 |
��3Fe+NaNO2+5NaOH�T3Na2FeO2+H2O+NH3��
��Na2FeO2+NaNO2+H2O�TNa2Fe2O4+NH3��+NaOH��δ��ƽ��
��Na2FeO2+Na2Fe2O4+2H2O�TFe3O4+4NaOH
����˵���в���ȷ���ǣ�������
| A�� | ��Ӧ����ƽ��H2O�Ļ�ѧ������Ϊ5 | |
| B�� | Fe3O4�ȿɿ�����������ֿɿ����������� | |
| C�� | ��Ӧ���У�Na2Fe2O4����������Na2FeO2�ǻ�ԭ�� | |
| D�� | ���������£�NaNO2�������Ա�Na2FeO2ǿ |
| A�� | �ϳɰ���Ӧ�У��Ͽ�1molN��N����ͬʱ����6molN-H�����ﻯѧƽ��״̬ | |
| B�� | ���������Ŀ��淴Ӧ�У�����Ӧ�����е�ѹǿ���ֲ���ʱ���ﵽ��ѧƽ��״̬ | |
| C�� | ����Ӧ����������ڻ�����еİٷֺ������ֲ���ʱ�����ﵽ��ѧƽ��״̬ | |
| D�� | ��ҵ�����в��ø�������Ϊ�����·�Ӧ���ת���ʱȵ���ʱ�� |
 ij����С��ģ�ҵ�Ʊ�����������£�
ij����С��ģ�ҵ�Ʊ�����������£�