��Ŀ����
(9��)Ԫ����������ָ������ѧϰԪ�ؼ��仯����֪ʶ����Ҫ���ߡ���֪����Ԫ�أ�����Po���IJ���֪ʶ���±���ʾ��
���ܽ����֪ʶ���ɣ�������Ԫ�������ɻش��������⣺
��1�����������۵㷶Χ������________________��
��2��Ԫ���ڵ���Ҫ���ϼۿ�����________________��
��3���������ڵ��⻯��ˮ��Һ��������ǿ������˳����________________(�û�ѧʽ��ʾ)��
��4���������н�ǿ��__________��������ԡ���ԭ�ԡ��������¶���ڿ����г��ڱ����ױ��ʣ�����ܷ�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
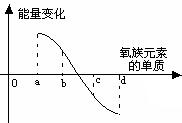
��5����ͼ��ʾΪ����Ԫ�ص�����H2��Ӧ�����е������仯ʾ��ͼ������a��b��c��d�ֱ��ʾ����Ԫ����ijһԪ�صĵ��ʣ�������Ϊ��ͬ���ʵ����ĵ�����H2��Ӧ�����е������仯�������仯��0��ʾ���ȣ������仯��0��ʾ���ȣ�����b����___________ _____�� d���� (��д��������)��
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵㣨�棩 | -218.4 | 113 | | 450 |
| ���ʷе㣨�棩 | -183 | 444.6 | 685 | 1390 |
| Ԫ����Ҫ���ϼ� | -2 | -2,+4,+6 | -2,+4,+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
��1�����������۵㷶Χ������________________��
��2��Ԫ���ڵ���Ҫ���ϼۿ�����________________��
��3���������ڵ��⻯��ˮ��Һ��������ǿ������˳����________________(�û�ѧʽ��ʾ)��
��4���������н�ǿ��__________��������ԡ���ԭ�ԡ��������¶���ڿ����г��ڱ����ױ��ʣ�����ܷ�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��5����ͼ��ʾΪ����Ԫ�ص�����H2��Ӧ�����е������仯ʾ��ͼ������a��b��c��d�ֱ��ʾ����Ԫ����ijһԪ�صĵ��ʣ�������Ϊ��ͬ���ʵ����ĵ�����H2��Ӧ�����е������仯�������仯��0��ʾ���ȣ������仯��0��ʾ���ȣ�����b����___________ _____�� d���� (��д��������)��

��1������113�棬С��450��(1��) ��2����2��+4��+6 (1��)
��3��H2Te��H2Se��H2S (2��) ��4����ԭ��(1��)��2H2Se+O2==2Se+2H2O(2��)
��5����(1��)����(1��)��
��3��H2Te��H2Se��H2S (2��) ��4����ԭ��(1��)��2H2Se+O2==2Se+2H2O(2��)
��5����(1��)����(1��)��
�����������1������������Ԫ���е����۵�ı仯��8O��52Te�����ߵĹ����еõ���
��2����ͬһ������Ԫ�ص���Ҫ���ϼ���ͬ���ڵ���Ҫ���ϼۿ����У�2��+4��+6��
��3����ͬһ�����У�Ԫ��ԭ������Խ��Ԫ�صķǽ�����Խ�����γɵ��⻯����ȶ���Խ��⻯��ˮ��Һ������Խǿ�����������ڵ��⻯��ˮ��Һ��������ǿ������˳����H2Te��H2Se��H2S��
��4����ͬһ�����У��γɵļ������ӵĻ�ԭ����ԭ�������������ǿ�����������н�ǿ�Ļ�ԭ�ԡ�
��5���ڴ˷�Ӧ�����зų�������Խ���⻯����ȶ���Խǿ���õ�a���⻯�����ȶ���d���⻯���ȶ������ɵá�

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ


 ���Ӷ����������Σ������ͬλ��ԭ�ӵ���������������֮��Ϊ
���Ӷ����������Σ������ͬλ��ԭ�ӵ���������������֮��Ϊ