��Ŀ����
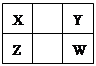
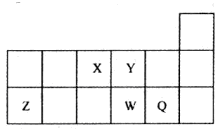
����������Ԫ��X��Y��Z��W��Q��ԭ��������������Xԭ�Ӻ��������������Ǵ�����2����Y�ķ�����YF3�����и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��Z��W�dz���������Z������������ͻ���ϣ�W�ļ�������ͬ���������Ӱ뾶��С�ģ�Q��p�ܼ�����һ��δ�ɶԵ��ӡ��Իش��������⣺
��1���Ƚϵ�һ�����ܣ�Z W�����������������������ͬ�����縺�ԣ�X Y��
��2��д��Q�ļ۵����Ų�ͼ ��YF3�Ľṹʽ ��
��3��������ͭ��Һ����ε���Y���⻯���ˮ��Һ�������������ӷ���ʽ��ʾ�ù��̳��ֵ�����仯��
_________________________ ��
��1���Ƚϵ�һ�����ܣ�Z W�����������������������ͬ�����縺�ԣ�X Y��
��2��д��Q�ļ۵����Ų�ͼ ��YF3�Ľṹʽ ��
��3��������ͭ��Һ����ε���Y���⻯���ˮ��Һ�������������ӷ���ʽ��ʾ�ù��̳��ֵ�����仯��
_________________________ ��
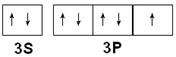
��1������ ���� ��2�� ��
�� 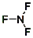 ��
��
��3��Cu2++2NH3��H2O = Cu(OH)2��+2NH4+��
Cu(OH)2+4NH3 = [Cu(NH3)4]2++2OH- ����Cu(OH)2+4NH3��H2O = [Cu(NH3)4]2++2OH-+4H2O��
 ��
�� 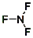 ��
����3��Cu2++2NH3��H2O = Cu(OH)2��+2NH4+��
Cu(OH)2+4NH3 = [Cu(NH3)4]2++2OH- ����Cu(OH)2+4NH3��H2O = [Cu(NH3)4]2++2OH-+4H2O��
�������������������Ԫ��X��Y��Z��W��Q��ԭ��������������Xԭ�Ӻ��������������Ǵ�����2���������������������ܳ���8������Xԭ����2�����Ӳ㣬����������Ϊ4����XΪ̼Ԫ�أ�Z��W�dz���������ԭ����������̼Ԫ�أ�����ǵ�������Ԫ�ء�Z������������ͻ���ϣ�W�ļ�������ͬ���������Ӱ뾶��С�ģ���WΪAlԪ�ء�ZΪMgԪ�أ�Y�ķ�����YF3�����и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ�����YΪ�ǽ�������Y����+3�ۣ�����������Ϊ8��3��5�����ڢ�A�壬ԭ������С��MgԪ�أ���YΪ��Ԫ�أ�Q���ڵ������ڣ�p�ܼ�����һ��δ�ɶԵ��ӣ���Χ�����Ų�Ϊ3s23p1��3s23p5������WΪAlԪ�أ���QΪClԪ�ء�
��1��������Խ������һ������Խ������Mgԭ��3s�ܼ�����2�����ӣ�Ϊȫ���ȶ�״̬�������ϵͣ���һ�����ܱ�AlԪ�صĸߣ�ͬ����������ҵ縺�������ʵ縺��C��N����
��2��QΪClԪ�أ���Χ�����Ų�Ϊ3s23p5��������Ԫ�صļ۵����Ų�ͼΪ
 ��NF3�е�ԭ�����ԭ��֮���γ�1�Թ��õ��Ӷԣ����ԽṹʽΪ��
��NF3�е�ԭ�����ԭ��֮���γ�1�Թ��õ��Ӷԣ����ԽṹʽΪ��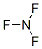 ��
����3��������ͭ��Һ����ε��백ˮ��Һ��������������������ͭ������泥�����ɫ�������ɡ������μӹ����İ�ˮ��������ͭ�백ˮ��Ӧ�����İ���ͭ�����ӣ���ɫ������ʧ����Һ������ɫ����Ӧ���ӷ���ʽΪ��Cu2++2NH3?H2O��Cu(OH)2��+2NH4+��Cu(OH)2+4NH3��H2O = [Cu(NH3)4]2++2OH-+4H2O��

��ϰ��ϵ�д�
�����Ŀ