��Ŀ����
����Ŀ�����������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʡ�
����������������ȡ����������������ͼ��ʾ��
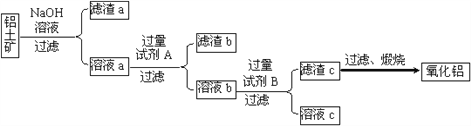
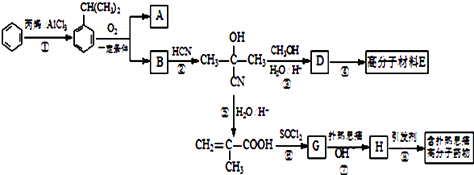
��1���Լ�A��________����Һb���Լ�B��Ӧ�����ӷ���ʽΪ________��
��2������NaOH��Һ���еķ�Ӧ�����ӷ���ʽΪ_________��____________��
���������մɵ����������������ַ����Ʊ�
��3�������������»�ԭ������Al2O3 +��C +�� N2 ![]() ����AlN +��CO������ƽ��
����AlN +��CO������ƽ��
���Ȼ����백�����ºϳɷ���AlCl3+NH3 ![]() AlN+3HCl
AlN+3HCl
��4�������ڱȷ������������ϸ������ơ�����˵���У���ȷ����_______��
A���������е� Al2O3��C��N2�ṹ�ȶ�����Ӧʱ�ƻ���ѧ����Ҫ���ĸ��������
B���������е�Al2O3��C���ײ����ڵ�������
C�����ַ����е�������Ϊ��ԭ����
���𰸡� ���ᣨ��������ᣩ Al3++3NH3��H2O =Al(OH)3��+3NH4+ Al2O3��2OH����2AlO2����H2O SiO2��2OH����SiO32����H2O 1 Al2O3 + 3 C + 1 N2 === 2 AlN + 3 CO AB
�����������������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʣ�����NaOH��Һ��Ӧ����Al2O3��SiO2����������a����Fe2O3����Һa����NaAlO2��Na2SiO3������Լ�AΪ������������ڳ�ȥNa2SiO3��������bΪH2SiO3����ҺbΪAlCl3���Լ�BΪ��ˮ���ǽ�AlCl3ת��ΪAl(OH)3��������������cΪAl(OH)3����ҺcΪNH4Cl��Al(OH)3���ȶ������շֽ�����Al2O3��
(1) �Լ�AΪ������������ڳ�ȥNa2SiO3����Һb���Լ�B��Ӧ�����ӷ���ʽΪAl3++ 3NH3��H2O =Al(OH)3��+3NH4+��
(2) ����NaOH��Һ��Ӧ����Al2O3��SiO2����Ӧ�����ӷ���ʽΪAl2O3��2OH�� �� 2AlO2�� ��H2O �� SiO2��2OH�� �� SiO32����H2O��
(3)��Ӧ��NԪ�ؽ���6����CԪ������2�ۣ�������ƽ��ķ���ʽΪAl2O3 +3C +N2 ===2AlN +3CO
(4)A���������е� Al2O3��C��N2�ṹ�ȶ������ʶ��ȷ������е�AlCl3��NH3�ȶ�����Ӧʱ�ƻ���ѧ����Ҫ���ĸ��������������A��ȷ��B��������״̬�Ͽ����������е�Al2O3��C���ǹ��������ײ����ڹ�����ﵪ�����У����������е�AlCl3��NH3�������¶������壬�����ڲ���ķ��뾻��������B��ȷ��C���ڷ�������NԪ�ػ��ϼ۽��ͣ�����ԭ����AlN������AlN�ǻ�ԭ������������Ƿ�������ԭ��Ӧ������C������ȷ��ΪAB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����1��Ũ������ľ̿���ڼ��������·�Ӧ�Ļ�ѧ����ʽΪ_______________________��
��� | �� | �� | �� | �� |
װ�� |
|
|
|
|
��2�����ñ�����ʾ��װ�����һ��ʵ��,��֤������Ӧ�������ĸ��ֲ��
��Щװ�õ�����˳��(���������������ҵķ���)��(��װ�õı��)����������������������������������������______________
��3��ʵ�����пɹ۲쵽װ�â���Aƿ����Һ��ɫ,Cƿ����Һ����ɫ��Bƿ��Һ��������___________��Cƿ��Һ��������________________��
��4��װ�â������ӵĹ���ҩƷ��__________��
��5��װ�â�����ʢ����Һ��___________������֤�IJ�����________��