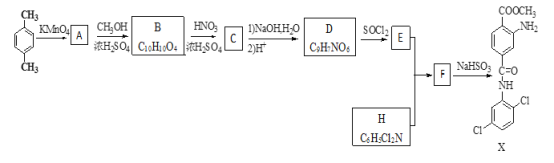
��Ŀ����
����Ŀ��������������þ��2.8 gͶ��100 mLϡ�����������ٲ�������Ϊֹ�����ռ�����״������2.24 L����������Һ�е���4.0 mol�qL-1������������Һ����ʼ����ʱ�������ɣ��μ�һ����������������Һ��ʼ���ְ�ɫ���������μ�����������Һ�����100 mLʱ��ǡ��ʹ������ȫ������(��Һ����仯���Բ���)��
(1)þ�����ѱ�������þ��δ��������þ�����ʵ���֮��____________��
(2)����������Һ�����ʵ����ʵ���Ũ��___________��
���𰸡�1:10 1.0 mol/L
��������
��1��þ��ϡ���ᷴӦ��������þ����������Ӧ�Ļ�ѧ����ʽΪMg+H2S04=MgSO4+H2������״�������£�2.24 L���������ʵ���Ϊ0.1mol���ɻ�ѧ����ʽ��֪þ�����ʵ���Ϊ0.1mol��������þ�����ʵ���Ϊ![]() =0.01mol����þԭ�Ӹ����غ��֪����������þ�����ʵ���Ϊ0.01mol��þ�����ѱ�������þ��δ��������þ�����ʵ���֮��Ϊ0.01mol��0.1mol=1:10���ʴ�Ϊ��1:10��
=0.01mol����þԭ�Ӹ����غ��֪����������þ�����ʵ���Ϊ0.01mol��þ�����ѱ�������þ��δ��������þ�����ʵ���֮��Ϊ0.01mol��0.1mol=1:10���ʴ�Ϊ��1:10��
��2���������֪�����������������Һ���к���Һ��δ��Ӧ��H2SO4��Ȼ����þ���ӷ�Ӧ����������þ������������þ������ȫʱNaOHȫ��ת��ΪNa2SO4������ԭ�Ӹ����غ��֪��n��Na2SO4��=![]() n��NaOH��=
n��NaOH��=![]() ��4.0 mol�qL-1��0.1L=0.2mol������������Һ�������Ƶ����ʵ���Ũ��
��4.0 mol�qL-1��0.1L=0.2mol������������Һ�������Ƶ����ʵ���Ũ��![]() =1.0mol/L���ʴ�Ϊ��1.0mol/L��
=1.0mol/L���ʴ�Ϊ��1.0mol/L��

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�