��Ŀ����
����Ŀ��A��B��C��D ����Ԫ�أ�ԭ��������������A ԭ�ӵ����������4�����ӣ�B �������Ӻ� C �������Ӿ�����ͬ�ĵ��Ӳ�ṹ����Ԫ�صĵ��ʷ�Ӧ������һ�ֵ���ɫ�Ĺ��� E��D �� L ����������� K��M �������Ӳ��ϵĵ�����֮�͡�
(1)C ���ӵĽṹʾ��ͼΪ_______ ��D �����ڱ���λ�� ______________��
(2)д�� E �ĵ���ʽ��______________ ��
(3)A��D ��Ԫ���γɵĻ������� ______________(��������������������)�����
(4)д�� D ������������ˮ����� A ���ʷ�Ӧ�Ļ�ѧ����ʽ��_______ ��
(5)B ԭ������ԭ���γɵ������У��� NH3 ������ͬ��������������Ϊ______________(�ѧʽ)�����������ӵĵ���ʽΪ______________ ��
(6)A��D ��Ԫ���γɵ�ij����������� CO2 ���ƵĽṹ�����õ���ʽ��ʾ���γɹ���__________________________________________ ��
���𰸡�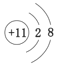 �������ڵ���A��
�������ڵ���A�� ![]() ���� C+2H2SO4(Ũ)
���� C+2H2SO4(Ũ)![]() CO2
CO2![]() +2SO2
+2SO2![]() +2H2O OH-
+2H2O OH- ![]()
![]()
��������
A�����������4�����ӣ���Aλ��IVA�壬B�������Ӻ�C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��Cλ��B����һ���ڣ���Ԫ�صĵ��ʷ�Ӧ������һ�ֵ���ɫ�Ĺ���E��EΪNa2O2���Ƴ�BΪO��CΪNa������Ԫ�ص�ԭ��������������AΪC�����ݺ�������Ų����ɣ��Ƴ�DΪS���ݴ˷�����
A�����������4�����ӣ���Aλ��IVA�壬B�������Ӻ�C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��Cλ��B����һ���ڣ���Ԫ�صĵ��ʷ�Ӧ������һ�ֵ���ɫ�Ĺ���E��EΪNa2O2���Ƴ�BΪO��CΪNa������Ԫ�ص�ԭ��������������AΪC�����ݺ�������Ų����ɣ��Ƴ�DΪS��
(1)CΪNa����������ΪNa�����ṹʾ��ͼΪ ��DΪS��λ�ڵ�������VIA�壻
��DΪS��λ�ڵ�������VIA�壻
(2)EΪNa2O2���������ӻ��������Na����O22����ɣ���Na2O2�ĵ���ʽΪ![]() ��
��
(3)C��S�γɵĻ�������CS2�����ڹ��ۻ����
(4)D������������ˮ����ΪH2SO4��C��Ũ�����ڼ��ȵ������£�����CO2��SO2������Ӧ����ʽΪC��2H2SO4(Ũ) ![]() CO2����2SO2����2H2O��
CO2����2SO2����2H2O��
(5)O��H�γɵ�����H2O2��H2O��OH����H3O����H2O2����18���ӣ�H2O��OH����H3O������10���ӣ�NH3Ϊ10����������NH3������ͬ����������������OH����OH���ĵ���ʽΪ![]() ��
��
(6)C��S�γɵĻ�������CS2��CO2�Ľṹ��ʽΪO=C=O�����CS2�Ľṹ��ʽΪS=C=S������ʽ��ʾ���γɹ�����![]() ��
��

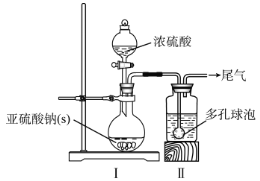
����Ŀ����ˮ���Ȼ���(SnCl4)�������л��ϳɵ��Ȼ�������ʵ���ҿ��������������Ʊ���ˮ���Ȼ�����ʵ��װ��ͼ��ͼ��
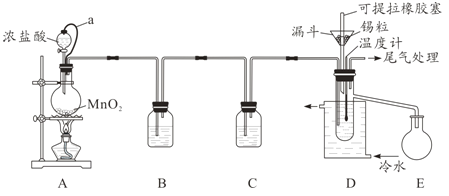
�������Ͽ�֪��
��Sn(s)+2Cl2(g)=SnCl4(l) ��H=-511kJ/mol
��SnCl4�ӷ���������ˮ�⡣
��������ʵ������������£�
���� | Sn | SnCl4 | CuCl2 |
�۵�/�� | 232 | -33 | 620 |
�е�/�� | 2260 | 114 | 993 |
�ܶ�/g��cm-3 | 7.310 | 2.226 | 3.386 |
�ش��������⣺
(1)a�ܵ�������__________��
(2)A�з�Ӧ�����ӷ���ʽ��__________��
(3)D����ȴˮ��������________________��
(4)β������ʱ����ѡ�õ�װ����__________(�����)��
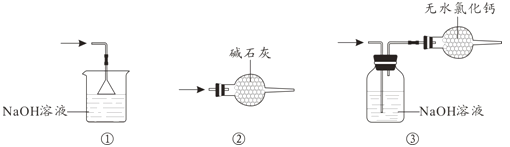
(5)�����к�ͭ������D�в���CuCl2������Ӱ��E�в�Ʒ�Ĵ��ȣ�ԭ����________��
(6)�Ƶõ�SnCl4��Ʒ�г�����SnCl2���������·����ⶨ��Ʒ���ȣ���ȷ����7.60g��Ʒ����ƿ�У��ټӹ�����FeCl3��Һ��������Ӧ��SnCl2+2FeCl3=SnCl4+2FeCl2������0.1000mol/LK2Cr2O7����Һ�ζ����ɵ�Fe2+����ʱ��ԭ����ΪCr3+�����ı���Һ20.00mL����SnCl4��Ʒ�Ĵ���Ϊ__________��