��Ŀ����
��2010?�����ģ�⣩�о���ѧ��Ӧ���������ķ�չ��������Ҫ���壮
��1����֪��N2��g��+3H2��g��2NH3��g����H1=-92.4kJ/mol
2H2��g��+O2��g��=2H2O��g����H2=-523.6kJ/mol
H2O��g��=H2O��l����H3=-44.0kJ/mol
�������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ���ɰ�������д���˷�Ӧ���Ȼ�ѧ����ʽ
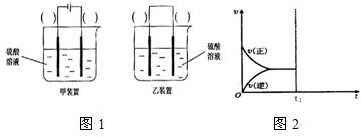
��2�����з�Ӧ��Cu+H2SO4�TCuSO4+H2����ͨ����ͼ1��ʾװ��ʵ��������Ӧ����ѡ����ʵ�װ������
���ڱ���缫���ϣ��Cu����C����������д��C�缫�ϵĵ缫��Ӧʽ
��3������ʹ�õĹ�¯��Ҫ���ڳ�ˮ��������ή��ȼ�ϵ������ʣ�ˮ���к��е�CaSO4��������Na2CO3��Һ������ʹ֮ת��Ϊ���ɡ����������CaCO3�����������ȥ����CaSO4 ת��ΪCaCO3�����ӷ���ʽΪ
��4����ѧ�о���������Cu2O�Ŀ���Ϊ̫����ֽ�ˮ�Ĵ�����һ���¶��£���2L�ܱ������м�������Cu2O��ͨ��0.10molˮ������������Ӧ��2H2O��g��
2H2��g��+O2��g����H��0����ͬʱ�β���O2�������±���
��
�ٸ�������������Ӧ�Ļ�ѧƽ�ⳣ��K=
�����ﵽƽ�����t1ʱ�̱����¶Ȳ��䣬�������������С������ͼ2�л��������淴Ӧ������ʱ��仯�Ĺ�ϵͼ��

��1����֪��N2��g��+3H2��g��2NH3��g����H1=-92.4kJ/mol
2H2��g��+O2��g��=2H2O��g����H2=-523.6kJ/mol
H2O��g��=H2O��l����H3=-44.0kJ/mol
�������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ���ɰ�������д���˷�Ӧ���Ȼ�ѧ����ʽ
2N2��g��+6H2O��l��=4NH3��g��+3O2��g����H=-1650kJ/mol
2N2��g��+6H2O��l��=4NH3��g��+3O2��g����H=-1650kJ/mol
����2�����з�Ӧ��Cu+H2SO4�TCuSO4+H2����ͨ����ͼ1��ʾװ��ʵ��������Ӧ����ѡ����ʵ�װ������
���ڱ���缫���ϣ��Cu����C����������д��C�缫�ϵĵ缫��Ӧʽ
2H++2e-��H2��
2H++2e-��H2��
����3������ʹ�õĹ�¯��Ҫ���ڳ�ˮ��������ή��ȼ�ϵ������ʣ�ˮ���к��е�CaSO4��������Na2CO3��Һ������ʹ֮ת��Ϊ���ɡ����������CaCO3�����������ȥ����CaSO4 ת��ΪCaCO3�����ӷ���ʽΪ
CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq��
CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq��
����4����ѧ�о���������Cu2O�Ŀ���Ϊ̫����ֽ�ˮ�Ĵ�����һ���¶��£���2L�ܱ������м�������Cu2O��ͨ��0.10molˮ������������Ӧ��2H2O��g��
| �� |
| Cu2O |
| ʱ��/min | 20 | 40 | 60 | 80 |
| n��O2��/mol | 0.0010 | 0.0016 | 0.0020 | 0.0020 |
�ٸ�������������Ӧ�Ļ�ѧƽ�ⳣ��K=
1.7��10-6mol/L
1.7��10-6mol/L
�������ﵽƽ�����t1ʱ�̱����¶Ȳ��䣬�������������С������ͼ2�л��������淴Ӧ������ʱ��仯�Ĺ�ϵͼ��
��������1�����ݸ�˹���������㻯ѧ��Ӧ���ʱ䣻
��2��Cu��H2SO4֮��ķ�Ӧ�Ƿ��Է��ģ���Ҫ����ʵ�֣����ݵ��صĹ����������ش�
��3���������Ÿ����ܵķ���ת����
��4���ٸ��ݻ�ѧƽ�ⳣ������ʽ��д�����ش�
�ڱ����¶Ȳ��䣬�������������С����ѹǿ�������淴Ӧ���ʾ�����ƽ���������������С�ķ�����У�
��2��Cu��H2SO4֮��ķ�Ӧ�Ƿ��Է��ģ���Ҫ����ʵ�֣����ݵ��صĹ����������ش�
��3���������Ÿ����ܵķ���ת����
��4���ٸ��ݻ�ѧƽ�ⳣ������ʽ��д�����ش�
�ڱ����¶Ȳ��䣬�������������С����ѹǿ�������淴Ӧ���ʾ�����ƽ���������������С�ķ�����У�
����⣨1����֪����N2��g��+3H2��g��2NH3��g����H1=-92.4kJ/mol
��2H2��g��+O2��g��=2H2O��g����H2=-523.6kJ/mol
��H2O��g��=H2O��l����H3=-44.0kJ/mol
��Ӧ2N2��g��+6H2O��l��=4NH3��g��+3O2��g�����ڢ١�2-3����-6���ۣ����ԡ�H=2��H1-3��H2-6��H3=-1650 kJ/mol��
�ʴ�Ϊ��2N2��g��+6H2O��l��=4NH3��g��+3O2��g����H=-1650 kJ/mol��
��2��Cu��H2SO4֮��ķ�Ӧ�Ƿ��Է��ģ���Ҫ����ʵ�֣�����ͭ�������������ǵ��弴�ɣ������Ϊ���ᣬ���� ��
��
C�缫Ϊ��������ӦΪ��2H++2e-��H2�����ʴ�Ϊ�� ��2H++2e-��H2����
��2H++2e-��H2����
��3���������Ÿ����ܵķ���ת����CaSO4��̼���Ʒ�Ӧת��ΪCaCO3�����ӷ���ʽΪ��CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����
�ʴ�Ϊ��CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����
��4���ٽ⣺���ݱ������ݣ����Կ�����Ӧ��60minʱ�ﵽƽ�⣬��
2H2O��g��
2H2��g��+O2��g��
��ʼŨ�ȣ�0.05 0 0
�仯Ũ�ȣ�0.002 0.002 0.001
ƽ��Ũ�ȣ�0.048 0.002 0.001
��ƽ�ⳣ��K=
=1.7��10-6mol/L����ͼ��֪H2��Ũ����ʱ�併�ͣ��ı�����˲��H2Ũ�Ȳ��䣬���Բ������¶Ȳ��䣬�������������С��
��ѹǿ�������淴Ӧ���ʾ�����ƽ���������������С�ķ�����У���������У������淴Ӧ���ʻ��Ǵ�������Ӧ���ʣ���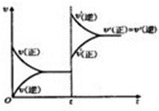 ��
��
�ʴ�Ϊ��1.7��10-6mol/L�� ��
��
��2H2��g��+O2��g��=2H2O��g����H2=-523.6kJ/mol
��H2O��g��=H2O��l����H3=-44.0kJ/mol
��Ӧ2N2��g��+6H2O��l��=4NH3��g��+3O2��g�����ڢ١�2-3����-6���ۣ����ԡ�H=2��H1-3��H2-6��H3=-1650 kJ/mol��
�ʴ�Ϊ��2N2��g��+6H2O��l��=4NH3��g��+3O2��g����H=-1650 kJ/mol��
��2��Cu��H2SO4֮��ķ�Ӧ�Ƿ��Է��ģ���Ҫ����ʵ�֣�����ͭ�������������ǵ��弴�ɣ������Ϊ���ᣬ����
 ��
��C�缫Ϊ��������ӦΪ��2H++2e-��H2�����ʴ�Ϊ��
 ��2H++2e-��H2����
��2H++2e-��H2������3���������Ÿ����ܵķ���ת����CaSO4��̼���Ʒ�Ӧת��ΪCaCO3�����ӷ���ʽΪ��CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����
�ʴ�Ϊ��CaSO4��s��+CO32-��aq��=CaCO3��s��+SO42-��aq����
��4���ٽ⣺���ݱ������ݣ����Կ�����Ӧ��60minʱ�ﵽƽ�⣬��
2H2O��g��
| �� |
| Cu2O |
��ʼŨ�ȣ�0.05 0 0
�仯Ũ�ȣ�0.002 0.002 0.001
ƽ��Ũ�ȣ�0.048 0.002 0.001
��ƽ�ⳣ��K=
| c2(H2)?c(O2) |
| c2(H2O) |
��ѹǿ�������淴Ӧ���ʾ�����ƽ���������������С�ķ�����У���������У������淴Ӧ���ʻ��Ǵ�������Ӧ���ʣ���
 ��
���ʴ�Ϊ��1.7��10-6mol/L��
 ��
�����������⿼��ѧ����ѧƽ�ⳣ���ĺ����Լ���ѧ��Ӧ���ʺͻ�ѧƽ���Ӱ������֪ʶ����һ���ۺ��⣬�ѶȽϴ�

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
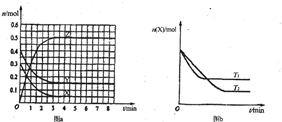 ��2010?�����ģ�⣩T0��ʱ����2L���ܱ������з�����Ӧ��aX��g��+bY��g��?cZ��g���������ʵ����ʵ�����ʱ��仯�Ĺ�ϵ��ͼa��ʾ������������ͬ���¶ȷֱ�ΪT1�桢T2��ʱ������Ӧ��X�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼb��ʾ��������������ȷ���ǣ�������
��2010?�����ģ�⣩T0��ʱ����2L���ܱ������з�����Ӧ��aX��g��+bY��g��?cZ��g���������ʵ����ʵ�����ʱ��仯�Ĺ�ϵ��ͼa��ʾ������������ͬ���¶ȷֱ�ΪT1�桢T2��ʱ������Ӧ��X�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼb��ʾ��������������ȷ���ǣ�������


