��Ŀ����
���������Ҫ�ɷֵĻ�ѧʽΪFeO��Cr2O3��������SiO2��Al2O3�����ʡ���ҵ�ϳ����ù�������������ø�������Ʊ��ظ���أ�����Ϊ��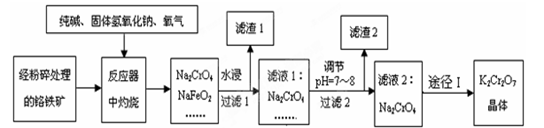
��֪����NaFeO2��ˮǿ��ˮ�⣻
���ظ����Ϊ�Ⱥ�ɫ��״���壬����ˮ���������Ҵ�����ǿ�����ԣ�
��2CrO42�� + 2H+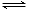 Cr2O72��+ H2O
Cr2O72��+ H2O
��1�����ղ���һ���������н��У����������ʺ�����������ʵ���������ϵ���
| A���� | B�������� | C��ʯӢ | D���մ� |
��д��Cr2O3�ʹ��������Ӧ����Na2CrO4�Ļ�ѧ����ʽΪ ��
��NaFeO2ˮ��ʱǿ��ˮ�����������������������ӷ���ʽΪ___ ___��
��3����Һ1�ijɷֳ�Na2CrO4��NaOH�⣬�����У��ѧʽ�� ��
��4��ͨ��;�����Na2CrO4��Һ���K2Cr2O7���壬���������У����ữ������KCl���������Ũ��������a�����ˡ�ϴ�ӡ����
�� ���ữ�������ô��������ҺpH��5����Ŀ���� ��
�ڲ���a������ ��
��1��A ( 3�֣�
��2���� ����Ӧ��ı�������ӿ췴Ӧ���ʣ�2�֣�
�� 2Cr2O3 + 3O2+4Na2CO3  4Na2CrO4+4CO2����3�֣�δ��ƽ��1�֣�
4Na2CrO4+4CO2����3�֣�δ��ƽ��1�֣�
�� FeO2��+2H2O=Fe(OH)3��+OH�� ��3�֣�δ��ƽ��1�֣�
��3��NaAlO2 ��Na2SiO3��2�֣�
��4����ʹ2CrO42�� + 2H+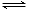 Cr2O72��+ H2O ��ƽ��������Ӧ�����ƶ���������CrO42-ת��ΪCr2O72-��2�֣�
Cr2O72��+ H2O ��ƽ��������Ӧ�����ƶ���������CrO42-ת��ΪCr2O72-��2�֣�
����ȴ�ᾧ��1�֣�ֻд��ȴ��ֻд�ᾧ���÷֣�
���������������1���մ��к��������裬ʯӢ�����óɷ��Ƕ������裬�������������������������跴Ӧ�������ʺ�����������ʵ���������ϵ���������ѡA��
��2���ٷ������ʹ���뷴Ӧ��ĽӴ�������ӿ췴Ӧ���ʣ�
�� ����Ԫ���غ��ж�Cr2O3�ʹ��������Ӧ����Na2CrO4�Ͷ�����̼����ѧ����ʽΪ
2Cr2O3 + 3O2+4Na2CO3  4Na2CrO4+4CO2����
4Na2CrO4+4CO2����
�� NaFeO2ˮ��ʱǿ��ˮ����������������������д���ӷ���ʽʱ������ű�Ϊ��=�������ӷ���ʽΪ
FeO2��+2H2O=Fe(OH)3��+OH��
��3��SiO2��Al2O3���������������Ʒ�Ӧ���ɹ����ơ�ƫ�����ƣ�������Һ1�ijɷֳ�Na2CrO4��NaOH�⣬������NaAlO2 ��Na2SiO3
��4���ٸ�����֪��Na2CrO4��Һ�д���2CrO42�� + 2H+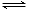 Cr2O72��+ H2Oƽ�⣬����������pH<5��ʹƽ�������ƶ���������CrO42-ת��ΪCr2O72-
Cr2O72��+ H2Oƽ�⣬����������pH<5��ʹƽ�������ƶ���������CrO42-ת��ΪCr2O72-
������Һ�õ������һ�㲽���Ǽ���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�������Ա�ʵ���м���Ũ����Ӧ��ȴ�ᾧ��
���㣺����������ѡ��ʵ�鲽����жϣ�������Ŀ��Ϣ��д��ѧ����ʽ�����ӷ���ʽ�����ʳɷֵ��ж�

����β���к���һ��������һ����̼���������������ʲ����Ĵ�����Ⱦ�ķ����ǰ�װ��ת������ʹ���Ƿ�����Ӧ����CO2��N2����Ӧ����ʽΪ��2CO��2NO2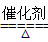 CO2��N2������NO2��
CO2��N2������NO2��
| A�������� | B����ԭ�� |
| C���������������ǻ�ԭ�� | D���Ȳ����������ֲ��ǻ�ԭ�� |
��(Tl)�����軯��(KCN)����ΪA��Σ��Ʒ����֪���з�Ӧ��һ���������ܹ�������
(1)Tl3����2Ag=Tl����2Ag��
(2)Ag����Fe2��=Ag��Fe3��
(3)Fe��2Fe3��=3Fe2����
�������������ԱȽ�˳����ȷ����
| A��Tl3��>Fe3��>Ag�� | B��Fe3��>Ag��>Tl3�� |
| C��Tl��>Ag��>Fe2�� | D��Tl3��>Ag��>Fe2�� |
2.8 g Feȫ������һ��Ũ�ȡ�200 mL��HNO3��Һ�У��õ���״���µ�����1.12 L����÷�Ӧ����Һ��pHΪ1������Ӧǰ����Һ����仯���Բ��ƣ��������й��ж���ȷ����
A����Ӧ����Һ��c(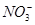 )��0.80 mol��L��1 )��0.80 mol��L��1 |
| B����Ӧ�����Һ�����ܽ�1.82 g Fe |
| C����ӦǰHNO3��Һ��Ũ��Ϊ1.0 mol��L��1 |
| D��1.12 L������NO��NO2�Ļ������ |
��51��2 g Cu��ȫ��������Ũ�����У��ռ�������������(��NO��N2O4��NO2)�Ļ���ﹲ0��8 mol����Щ����ǡ���ܱ�500 mL 2 mol/L NaOH��Һ��ȫ���գ������ķ�ӦΪ��2NO2��2NaOH=NaNO2��NaNO3��H2O��NO��NO2��2NaOH=2NaNO2��H2O��
�����ɵ�����Һ��NaNO2�����ʵ���Ϊ
| A��0��4 mol | B��0��6 mol |
| C��0��8 mol | D��0��2 mol |
��һ������Fe��Fe2O3�Ļ�������250 mL��1.8 mol��L��1��HNO3��Һ�У�������������ȫ�ܽ���ڱ�״��������1.12 L NO(HNO3�Ļ�ԭ�������һ��)������Ӧ�����Һ�м���1.0 mol��L��1 NaOH��Һ����Ҫʹ��Ԫ����ȫ�������������NaOH��Һ���������ӦΪ
| A��300 mL | B��400 mL | C��450 mL | D��500 mL |
ij��ȤС��̽��SO2���廹ԭFe3��������ʹ�õ�ҩƷ��װ������ͼ��ʾ������˵������������(����)��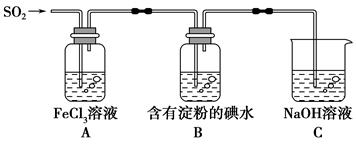
| A���ܱ���I���Ļ�ԭ������SO2��������B����ɫ��Һ��ɫ |
| B��װ��C������������SO2β������ֹ��Ⱦ���� |
| C��Ϊ����֤A�з�����������ԭ��Ӧ��������ϡ�����ữ��BaCl2��Һ��������ɫ���� |
| D��Ϊ����֤A�з�����������ԭ��Ӧ������KMnO4��Һ���Ϻ�ɫ��ȥ |
������һ����Ҫ�Ĺ�ҵԭ�ϡ���ҵ�����÷�Ӧ3Cl2��2NH3=N2��6HCl��������ܵ��Ƿ�©��������˵��������ǣ� ��
| A�����ܵ�©�������ͻ�������� |
| B���÷�Ӧ������Cl2��ǿ������ |
| C���÷�Ӧ���ڸ��ֽⷴӦ |
| D������1 mol N2��6 mol����ת�� |